Sec22b determines Weibel-Palade body length by controlling anterograde ER-Golgi transport
- PMID: 32336681
- PMCID: PMC8018124
- DOI: 10.3324/haematol.2019.242727
Sec22b determines Weibel-Palade body length by controlling anterograde ER-Golgi transport
Abstract
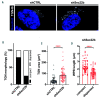
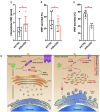
Von Willebrand factor (VWF) is a multimeric hemostatic protein that is synthesized in endothelial cells, where it is stored for secretion in elongated secretory organelles, so-called Weibel-Palade bodies (WPBs). Hemostatic activity of VWF is strongly tied to WPB length, but how endothelial cells control the dimensions of their WPBs is unclear. In this study we used a targeted shRNA screen to identify the longin-SNARE Sec22b as a novel determinant of WPB size and VWF trafficking. We found that Sec22b depletion resulted in loss of the typically elongated WPB morphology along with disintegration of the Golgi and dilation of rough ER (rER) cisternae. This was accompanied by reduced proteolytic processing of VWF, accumulation of VWF in the dilated rER and reduced basal and stimulated VWF secretion. Our data demonstrate that the elongation of WPBs, and thus adhesive activity of its cargo VWF, is determined by the rate of anterograde transport between ER and Golgi, which depends on Sec22b-containing SNARE complexes.
Figures



Similar articles
-
Syntaxin 5 determines Weibel-Palade body size and von Willebrand factor secretion by controlling Golgi architecture.Haematologica. 2022 Aug 1;107(8):1827-1839. doi: 10.3324/haematol.2021.280121. Haematologica. 2022. PMID: 35081689 Free PMC article.
-
Weibel-Palade Body Localized Syntaxin-3 Modulates Von Willebrand Factor Secretion From Endothelial Cells.Arterioscler Thromb Vasc Biol. 2018 Jul;38(7):1549-1561. doi: 10.1161/ATVBAHA.117.310701. Epub 2018 Jun 7. Arterioscler Thromb Vasc Biol. 2018. PMID: 29880488 Free PMC article.
-
Interaction networks of Weibel-Palade body regulators syntaxin-3 and syntaxin binding protein 5 in endothelial cells.J Proteomics. 2019 Aug 15;205:103417. doi: 10.1016/j.jprot.2019.103417. Epub 2019 Jun 13. J Proteomics. 2019. PMID: 31201948
-
Functional architecture of Weibel-Palade bodies.Blood. 2011 May 12;117(19):5033-43. doi: 10.1182/blood-2010-09-267492. Epub 2011 Jan 25. Blood. 2011. PMID: 21266719 Free PMC article. Review.
-
Lifecycle of Weibel-Palade bodies.Hamostaseologie. 2017 Jan 31;37(1):13-24. doi: 10.5482/HAMO-16-07-0021. Epub 2016 Dec 22. Hamostaseologie. 2017. PMID: 28004844 Review.
Cited by
-
Syntaxin 5 determines Weibel-Palade body size and von Willebrand factor secretion by controlling Golgi architecture.Haematologica. 2022 Aug 1;107(8):1827-1839. doi: 10.3324/haematol.2021.280121. Haematologica. 2022. PMID: 35081689 Free PMC article.
-
Altered Storage and Function of von Willebrand Factor in Human Cardiac Microvascular Endothelial Cells Isolated from Recipient Transplant Hearts.Int J Mol Sci. 2023 Feb 25;24(5):4553. doi: 10.3390/ijms24054553. Int J Mol Sci. 2023. PMID: 36901985 Free PMC article.
-
Weibel Palade Bodies: Unique Secretory Organelles of Endothelial Cells that Control Blood Vessel Homeostasis.Front Cell Dev Biol. 2021 Dec 16;9:813995. doi: 10.3389/fcell.2021.813995. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34977047 Free PMC article. Review.
-
Sec22b is a critical and nonredundant regulator of plasma cell maintenance.Proc Natl Acad Sci U S A. 2023 Jan 10;120(2):e2213056120. doi: 10.1073/pnas.2213056120. Epub 2023 Jan 3. Proc Natl Acad Sci U S A. 2023. PMID: 36595686 Free PMC article.
-
Weibel-Palade bodies - secretory organelles at the interface of inflammation and hemostasis.Front Cell Dev Biol. 2025 Jun 19;13:1624487. doi: 10.3389/fcell.2025.1624487. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40612111 Free PMC article. Review.
References
-
- Kienzle C, von Blume J. Secretory cargo sorting at the trans-Golgi network. Trends Cell Biol. 2014;24(10):584-593. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

