Probucol Trial for Secondary Prevention of Atherosclerotic Events in Patients with Coronary Heart Disease (PROSPECTIVE)
- PMID: 32336695
- PMCID: PMC7957028
- DOI: 10.5551/jat.55327
Probucol Trial for Secondary Prevention of Atherosclerotic Events in Patients with Coronary Heart Disease (PROSPECTIVE)
Abstract
Aims: Although intensive statin therapy reduced cardiovascular risks, cardiovascular events have not been completely prevented. Probucol is a potent antioxidant and reduces tendon xanthomas in familial hypercholesterolemia patients despite reduction of high-density lipoprotein (HDL)-cholesterol (HDL-C). We investigated whether probucol can reduce cardiovascular events on top of conventional lipid-lowering therapy in patients with coronary heart disease (CHD).
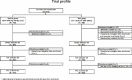
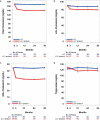
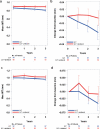
Methods: PROSPECTIVE is a multicenter, randomized, prospective study that recruited 876 Japanese patients with CHD and dyslipidemia with a low-density lipoprotein (LDL)-cholesterol (LDL-C) level of ≥ 140 mg/dL without medication or those treated with lipid-lowering drugs. Lipid-lowering agents were administered during the study period in the control group (n=438), and probucol 500 mg/day was added to lipid-lowering therapy in the probucol group (n=438). Patients were randomly assigned to two treatment groups by adjusting the LDL-C level and presence of diabetes and hypertension and followed up for more than 3 years. The primary end point was a composite of cerebrovascular and cardiovascular events (cardiovascular disease death including sudden death, nonfatal myocardial infarction, nonfatal stroke, hospitalization for unstable angina, hospitalization for heart failure, or coronary revascularization). The secondary end point was carotid intima-media thickness in a subset of patients.
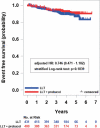
Results: The incidence of the primary end point showed a trend to be lower in the probucol group compared with that in the control group despite reduced HDL-C without serious adverse events. Anti-atherogenic effects of probucol may be attributed to its potent antioxidative function and enhancement of reverse cholesterol transport.
Conclusion: Since there was no statistical significance between the probucol and control groups despite a marked reduction of HDL-C, further studies on the clinical outcomes of probucol on top of conventional therapy may be necessary in the future (UMIN000003307).
Keywords: Antioxidants; Coronary heart disease; Prevention; Probucol; Reverse cholesterol transport.
Conflict of interest statement
Dr. Yamashita reports grants and personal fees from Kowa Company, Ltd., Bayer Yakuhin, Ltd., MSD K.K., Takeda Pharmaceutical Company, Ltd., Astellas Pharma Inc., grants from Kyowa Medex Co., Ltd., Hayashibara Co., Ltd., and personal fees from Skylight Biotec, Inc., Astellas Amgen, Sanofi, Aegerion, outside the submitted work. Dr. Arai reports personal fees from MSD, Pfizer, Kowa, Daiichi Sankyo, Astellas, during the conduct of the study. Dr. Bujo has nothing to disclose. Dr. Masuda reports grants and personal fees from Nippon Boehringer Ingelheim Co., Ltd., MSD K.K., Takeda Pharmaceutical Company, Ltd., Daiichi-Sankyo Company, Ltd., Mochida Pharmaceutical Company, Ltd., Kowa Company Ltd., Kowa Company Ltd., Kissei Pharmaceutical Co., Ltd.; grants from Otsuka Pharmaceutical Co Ltd.; personal fees from Kowa Company, Ltd., Bayer Yakuhin, Ltd, , Kyowa Medex Co., Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., Ono Pharmaceutical Company, Ltd., Astellas Pharma Inc., AstraZeneca K.K., non-financial support from Skylight Biotec, Inc., Pfizer Japan Inc., Amgen Astellas Biopharma K.K., Sanofi K.K., , grants from Shionogi & Co., Ltd., grants from Bayer Yakuhin, Ltd., grants from Sanwa Kagaku Kenkyusho Co., Ltd., grants from Astellas Pharma Inc., grants from Hayashibara Co., Ltd., Teijin Pharma Limited, Kaken Pharmaceutical Co., Ltd., outside the submitted work. Dr. Ohama reports grants from Daiichi Sankyo, MSD, outside the submitted work. Dr. Ishibashi and Dr. Yanagi have nothing to disclose. Dr. Doi is now also an employee (an occupational health physician) of Takeda Pharmaceutical Company Limited, Osaka, Japan. Mr. Nakagawa, Mr. Yamashiro, Mr. Tanabe, Dr. Kita, Dr. Matsuzaki, Dr. Saito and Dr. Fukushima have nothing to disclose. Dr. Matsuzawa reports personal fees from Teijin Pharma, Kowa, Sumitomo Dainippon Pharma, outside the submitted work.
Figures
References
-
- Cholesterol Treatment Trialists' (CTT) Collaboration, Fulcher J, O'Connell R, Voysey M, Emberson J, Blackwell L, Mihaylova B, Simes J, Collins R, Kirby A, Colhoun H, Braunwald E, La Rosa J, Pedersen TR, Tonkin A, Davis B, Sleight P, Franzosi MG, Baigent C, Keech A: Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet, 2015; 385: 1397-1405 - PubMed
-
- Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM; IMPROVE-IT Investigators: Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med, 2015; 372: 2387-2397 - PubMed
-
- Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR; FOURIER Steering Committee and Investigators: Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med, 2017; 4; 376: 1713-1722 - PubMed
-
- Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Lecorps G, Mahaffey KW, Moryusef A, Pordy R, Quintero K, Roe MT, Sasiela WJ, Tamby JF, Tricoci P, White HD, Zeiher AM; ODYSSEY OUTCOMES Committees and Investigators: Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med, 2018; 379: 2097-2107 - PubMed
References of Appendix
-
- Marks D, Thorogood M, Neil HA, Humphries SE: A review on the diagnosis, natural history, and treatment of familial hypercholesterolaemia. Atherosclerosis, 2003; 168: 1-14 - PubMed
-
- Sawayama Y, Shimizu C, Maeda N, Tatsukawa M, Kinukawa N, Koyanagi S, Kashiwagi S, Hayashi J: Effects of probucol and pravastatin on common carotid atherosclerosis in patients with asymptomatic hypercholesterolemia. Fukuoka Atherosclerosis Trial (FAST). J Am Coll Cardiol, 2002; 39: 610-616 - PubMed
-
- Yamashita S, Bujo H, Arai H, Harada-Shiba M, Matsui S, Fukushima M, Saito Y, Kita T, Matsuzawa Y: Long-term probucol treatment prevents secondary cardiovascular events: a cohort study of patients with heterozygous familial hypercholesterolemia in Japan. J Atheroscler Thromb, 2008; 15: 292-303 - PubMed
-
- Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K; Japan EPA lipid intervention study (JELIS) Investigators: Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet, 2007; 369: 1090-1098 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

