A Mobile Health Solution Complementing Psychopharmacology-Supported Smoking Cessation: Randomized Controlled Trial
- PMID: 32338624
- PMCID: PMC7215523
- DOI: 10.2196/17530
A Mobile Health Solution Complementing Psychopharmacology-Supported Smoking Cessation: Randomized Controlled Trial
Abstract
Background: Smoking cessation is a persistent leading public health challenge. Mobile health (mHealth) solutions are emerging to improve smoking cessation treatments. Previous approaches have proposed supporting cessation with tailored motivational messages. Some managed to provide short-term improvements in smoking cessation. Yet, these approaches were either static in terms of personalization or human-based nonscalable solutions. Additionally, long-term effects were neither presented nor assessed in combination with existing psychopharmacological therapies.
Objective: This study aimed to analyze the long-term efficacy of a mobile app supporting psychopharmacological therapy for smoking cessation and complementarily assess the involved innovative technology.
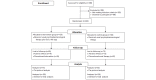
Methods: A 12-month, randomized, open-label, parallel-group trial comparing smoking cessation rates was performed at Virgen del Rocío University Hospital in Seville (Spain). Smokers were randomly allocated to a control group (CG) receiving usual care (psychopharmacological treatment, n=120) or an intervention group (IG) receiving psychopharmacological treatment and using a mobile app providing artificial intelligence-generated and tailored smoking cessation support messages (n=120). The secondary objectives were to analyze health-related quality of life and monitor healthy lifestyle and physical exercise habits. Safety was assessed according to the presence of adverse events related to the pharmacological therapy. Per-protocol and intention-to-treat analyses were performed. Incomplete data and multinomial regression analyses were performed to assess the variables influencing participant cessation probability. The technical solution was assessed according to the precision of the tailored motivational smoking cessation messages and user engagement. Cessation and no cessation subgroups were compared using t tests. A voluntary satisfaction questionnaire was administered at the end of the intervention to all participants who completed the trial.
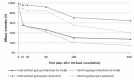
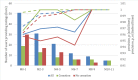
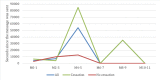
Results: In the IG, abstinence was 2.75 times higher (adjusted OR 3.45, P=.01) in the per-protocol analysis and 2.15 times higher (adjusted OR 3.13, P=.002) in the intention-to-treat analysis. Lost data analysis and multinomial logistic models showed different patterns in participants who dropped out. Regarding safety, 14 of 120 (11.7%) IG participants and 13 of 120 (10.8%) CG participants had 19 and 23 adverse events, respectively (P=.84). None of the clinical secondary objective measures showed relevant differences between the groups. The system was able to learn and tailor messages for improved effectiveness in supporting smoking cessation but was unable to reduce the time between a message being sent and opened. In either case, there was no relevant difference between the cessation and no cessation subgroups. However, a significant difference was found in system engagement at 6 months (P=.04) but not in all subsequent months. High system appreciation was reported at the end of the study.
Conclusions: The proposed mHealth solution complementing psychopharmacological therapy showed greater efficacy for achieving 1-year tobacco abstinence as compared with psychopharmacological therapy alone. It provides a basis for artificial intelligence-based future approaches.
Trial registration: ClinicalTrials.gov NCT03553173; https://clinicaltrials.gov/ct2/show/NCT03553173.
International registered report identifier (irrid): RR2-10.2196/12464.
Keywords: behavioral change; health recommender systems; mHealth; randomized controlled trial; smoking cessation.
©Laura Carrasco-Hernandez, Francisco Jódar-Sánchez, Francisco Núñez-Benjumea, Jesús Moreno Conde, Marco Mesa González, Antón Civit-Balcells, Santiago Hors-Fraile, Carlos Luis Parra-Calderón, Panagiotis D Bamidis, Francisco Ortega-Ruiz. Originally published in JMIR mHealth and uHealth (http://mhealth.jmir.org), 27.04.2020.
Conflict of interest statement
Conflicts of Interest: SHF is a product manager at Salumedia Tecnologías SLU, the company with exploitation rights to the digital therapeutic solution used in the So-Lo-Mo study (DigiQuit). He contributed to technical data extraction for the precision, time-to-open the messages, and perceived quality metrics and their analysis. He was not involved in the clinical trial design, execution, or analysis of the resulting clinical data. Some institutions of all the other authors (University of Seville, the Aristotle University of Thessaloniki, and the Servicio Andaluz de Salud as a legal representative institution for the Virgen del Rocío University Hospital) signed an exploitation agreement with Salumedia Tecnologías SLU to benefit from DigiQuit commercialization. This agreement was elaborated and signed before publishing the present results but after the trial was finished and its results were generated.
Figures

References
-
- WHO . WHO Global Report On Trends In Prevalence Of Tobacco Smoking, 2015. Geneva: World Health Organization; 2015.
-
- Reid RD, Pritchard G, Walker K, Aitken D, Mullen K, Pipe AL. Managing smoking cessation. CMAJ. 2016 Dec 06;188(17-18):E484–E492. doi: 10.1503/cmaj.151510. http://www.cmaj.ca/cgi/pmidlookup?view=long&pmid=27698200 cmaj.151510 - DOI - PMC - PubMed
-
- Cahill K, Lindson-Hawley N, Thomas K, Fanshawe T, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2016 May 09;(5):CD006103. doi: 10.1002/14651858.CD006103.pub7. http://europepmc.org/abstract/MED/27158893 - DOI - PMC - PubMed

