Myofibroblast contraction is essential for generating and regenerating the gas-exchange surface
- PMID: 32338642
- PMCID: PMC7260039
- DOI: 10.1172/JCI132189
Myofibroblast contraction is essential for generating and regenerating the gas-exchange surface
Abstract
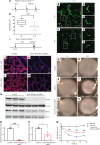
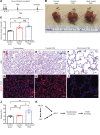
A majority (~95%) of the gas-exchange surface area is generated through septa formation during alveologenesis. Disruption of this process leads to alveolar simplification and bronchopulmonary dysplasia (BPD), a prevalent disorder in premature infants. Although several models have been proposed, the mechanism of septa formation remains under debate. Here we show that inactivation of myosin light chain kinase (MLCK), a key factor required for myofibroblast contraction, disrupted septa formation, supporting the myofibroblast contraction model of alveologenesis. The alveoli simplification phenotype was accompanied by decreased yes-associated protein (YAP), a key effector in the Hippo mechanotransduction pathway. Expression of activated YAP in Mlck-mutant lungs led to partial reversal of alveolar simplification. In the adult, although Mlck inactivation did not lead to simplification, it prevented reseptation during compensatory regrowth in the pneumonectomy model. These findings revealed that myofibroblast reactivation and contraction are requisite steps toward regenerating the gas-exchange surface in diseases such as BPD and chronic obstructive pulmonary disease (COPD).
Keywords: Development; Mouse models; Respiration.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

