CO-RADS: A Categorical CT Assessment Scheme for Patients Suspected of Having COVID-19-Definition and Evaluation
- PMID: 32339082
- PMCID: PMC7233402
- DOI: 10.1148/radiol.2020201473
CO-RADS: A Categorical CT Assessment Scheme for Patients Suspected of Having COVID-19-Definition and Evaluation
Abstract
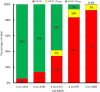
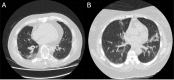
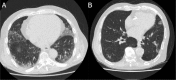
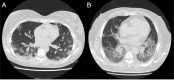
Background A categorical CT assessment scheme for suspicion of pulmonary involvement of coronavirus disease 2019 (COVID-19 provides a basis for gathering scientific evidence and improved communication with referring physicians. Purpose To introduce the COVID-19 Reporting and Data System (CO-RADS) for use in the standardized assessment of pulmonary involvement of COVID-19 on unenhanced chest CT images and to report its initial interobserver agreement and performance. Materials and Methods The Dutch Radiological Society developed CO-RADS based on other efforts for standardization, such as the Lung Imaging Reporting and Data System or Breast Imaging Reporting and Data System. CO-RADS assesses the suspicion for pulmonary involvement of COVID-19 on a scale from 1 (very low) to 5 (very high). The system is meant to be used in patients with moderate to severe symptoms of COVID-19. The system was evaluated by using 105 chest CT scans of patients admitted to the hospital with clinical suspicion of COVID-19 and in whom reverse transcription-polymerase chain reaction (RT-PCR) was performed (mean, 62 years ± 16 [standard deviation]; 61 men, 53 with positive RT-PCR results). Eight observers used CO-RADS to assess the scans. Fleiss κ value was calculated, and scores of individual observers were compared with the median of the remaining seven observers. The resulting area under the receiver operating characteristics curve (AUC) was compared with results from RT-PCR and clinical diagnosis of COVID-19. Results There was absolute agreement among observers in 573 (68.2%) of 840 observations. Fleiss κ value was 0.47 (95% confidence interval [CI]: 0.45, 0.47), with the highest κ value for CO-RADS categories 1 (0.58, 95% CI: 0.54, 0.62) and 5 (0.68, 95% CI: 0.65, 0.72). The average AUC was 0.91 (95% CI: 0.85, 0.97) for predicting RT-PCR outcome and 0.95 (95% CI: 0.91, 0.99) for clinical diagnosis. The false-negative rate for CO-RADS 1 was nine of 161 cases (5.6%; 95% CI: 1.0%, 10%), and the false-positive rate for CO-RADS category 5 was one of 286 (0.3%; 95% CI: 0%, 1.0%). Conclusion The coronavirus disease 2019 (COVID-19) Reporting and Data System (CO-RADS) is a categorical assessment scheme for pulmonary involvement of COVID-19 at unenhanced chest CT that performs very well in predicting COVID-19 in patients with moderate to severe symptoms and has substantial interobserver agreement, especially for categories 1 and 5. © RSNA, 2020 Online supplemental material is available for this article.
Figures
Comment in
-
Extensive pulmonary perfusion defects compatible with microthrombosis and thromboembolic disease in severe Covid-19 pneumonia.Thromb Res. 2020 Dec;196:135-137. doi: 10.1016/j.thromres.2020.08.026. Epub 2020 Aug 22. Thromb Res. 2020. PMID: 32866825 Free PMC article. No abstract available.
References
-
- Li Y, Yao L, Li J, et al. Stability issues of RT-PCR testing of SARS-CoV-2 for hospitalized patients clinically diagnosed with COVID-19. J Med Virol. 2020;http://doi.wiley.com/10.1002/jmv.25786. Accessed April 15, 2020. - DOI - PMC - PubMed
-
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020;https://jamanetwork.com/journals/jama/fullarticle/2762997. Accessed April 15, 2020. - PMC - PubMed
-
- Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25(3); https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2020.25.3..... Accessed April 16, 2020. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

