Metabolic insights from a GHSR-A203E mutant mouse model
- PMID: 32339772
- PMCID: PMC7242877
- DOI: 10.1016/j.molmet.2020.101004
Metabolic insights from a GHSR-A203E mutant mouse model
Abstract
Objective: Binding of ghrelin to its receptor, growth hormone secretagogue receptor (GHSR), stimulates GH release, induces eating, and increases blood glucose. These processes may also be influenced by constitutive (ghrelin-independent) GHSR activity, as suggested by findings in short people with naturally occurring GHSR-A204E mutations and reduced food intake and blood glucose in rodents administered GHSR inverse agonists, both of which impair constitutive GHSR activity. In this study, we aimed to more fully determine the physiologic relevance of constitutive GHSR activity.
Methods: We generated mice with a GHSR mutation that replaces alanine at position 203 with glutamate (GHSR-A203E), which corresponds to the previously described human GHSR-A204E mutation, and used them to conduct ex vivo neuronal electrophysiology and in vivo metabolic assessments. We also measured signaling within COS-7 and HEK293T cells transfected with wild-type GHSR (GHSR-WT) or GHSR-A203E constructs.
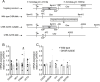
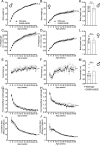
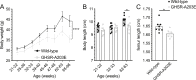
Results: In COS-7 cells, GHSR-A203E resulted in lower baseline IP3 accumulation than GHSR-WT; ghrelin-induced IP3 accumulation was observed in both constructs. In HEK293T cells co-transfected with voltage-gated CaV2.2 calcium channel complex, GHSR-A203E had no effect on basal CaV2.2 current density while GHSR-WT did; both GHSR-A203E and GHSR-WT inhibited CaV2.2 current in the presence of ghrelin. In cultured hypothalamic neurons from GHSR-A203E and GHSR-deficient mice, native calcium currents were greater than those in neurons from wild-type mice; ghrelin inhibited calcium currents in cultured hypothalamic neurons from both GHSR-A203E and wild-type mice. In brain slices, resting membrane potentials of arcuate NPY neurons from GHSR-A203E mice were hyperpolarized compared to those from wild-type mice; the same percentage of arcuate NPY neurons from GHSR-A203E and wild-type mice depolarized upon ghrelin exposure. The GHSR-A203E mutation did not significantly affect body weight, body length, or femur length in the first ∼6 months of life, yet these parameters were lower in GHSR-A203E mice after 1 year of age. During a 7-d 60% caloric restriction regimen, GHSR-A203E mice lacked the usual marked rise in plasma GH and demonstrated an exaggerated drop in blood glucose. Administered ghrelin also exhibited reduced orexigenic and GH secretagogue efficacies in GHSR-A203E mice.
Conclusions: Our data suggest that the A203E mutation ablates constitutive GHSR activity and that constitutive GHSR activity contributes to the native depolarizing conductance of GHSR-expressing arcuate NPY neurons. Although the A203E mutation does not block ghrelin-evoked signaling as assessed using in vitro and ex vivo models, GHSR-A203E mice lack the usual acute food intake response to administered ghrelin in vivo. The GHSR-A203E mutation also blunts GH release, and in aged mice leads to reduced body length and femur length, which are consistent with the short stature of human carriers of the GHSR-A204E mutation.
Keywords: Constitutive activity; GH; Ghrelin; Growth hormone.
Copyright © 2020 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Figures






References
-
- Kojima M., Hosoda H., Date Y., Nakazato M., Matsuo H., Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. - PubMed
-
- Holst B., Schwartz T.W. Constitutive ghrelin receptor activity as a signaling set-point in appetite regulation. Trends in Pharmacological Sciences. 2004;25:113–117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

