Blockade of CDK7 Reverses Endocrine Therapy Resistance in Breast Cancer
- PMID: 32340192
- PMCID: PMC7215326
- DOI: 10.3390/ijms21082974
Blockade of CDK7 Reverses Endocrine Therapy Resistance in Breast Cancer
Abstract
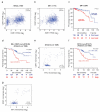
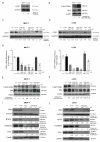
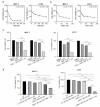
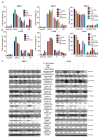
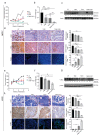
Cyclin-dependent kinase (CDK)-7 inhibitors are emerging as promising drugs for the treatment of different types of cancer that show chemotherapy resistance. Evaluation of the effects of CDK7 inhibitor, THZ1, alone and combined with tamoxifen is of paramount importance. Thus, in the current work, we assessed the effects of THZ1 and/or tamoxifen in two estrogen receptor-positive (ER+) breast cancer cell lines (MCF7) and its tamoxifen resistant counterpart (LCC2) in vitro and in xenograft mouse models of breast cancer. Furthermore, we evaluated the expression of CDK7 in clinical samples from breast cancer patients. Cell viability, apoptosis, and genes involved in cell cycle regulation and tamoxifen resistance were determined. Tumor volume and weight, proliferation marker (Ki67), angiogenic marker (CD31), and apoptotic markers were assayed. Bioinformatic data indicated CDK7 expression was associated with negative prognosis, enhanced pro-oncogenic pathways, and decreased response to tamoxifen. Treatment with THZ1 enhanced tamoxifen-induced cytotoxicity, while it inhibited genes involved in tumor progression in MCF-7 and LCC2 cells. In vivo, THZ1 boosted the effect of tamoxifen on tumor weight and tumor volume, reduced Ki67 and CD31 expression, and increased apoptotic cell death. Our findings identify CDK7 as a possible therapeutic target for breast cancer whether it is sensitive or resistant to tamoxifen therapy.
Keywords: breast cancer; c-Myc; cyclin-dependent kinase 7; estrogen receptor; resistance; tamoxifen.
Conflict of interest statement
The authors declare that they have no competing interests. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

