Three-Component Access to Functionalized Spiropyrrolidine Heterocyclic Scaffolds and Their Cholinesterase Inhibitory Activity
- PMID: 32340203
- PMCID: PMC7221748
- DOI: 10.3390/molecules25081963
Three-Component Access to Functionalized Spiropyrrolidine Heterocyclic Scaffolds and Their Cholinesterase Inhibitory Activity
Abstract
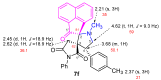
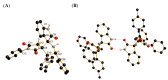
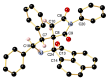
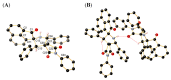
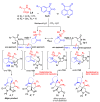
A novel one-pot [3+2]-cycloaddition reaction of (E)-3-arylidene-1-phenyl-succinimides, cyclic 1,2-diketones (isatin, 5-chloro-isatin and acenaphtenequinone), and diverse α-aminoacids such as 2-phenylglycine or sarcosine is reported. The reaction provides succinimide-substituted dispiropyrrolidine derivatives with high regio- and diastereoselectivities under mild reaction conditions. The stereochemistry of these N-heterocycles has been confirmed by four X-ray diffraction studies. Several synthetized compounds show higher inhibition on acetylcholinesterase (AChE) than butyrylcholinesterase (BChE). Of the 17 synthesized compounds tested, five exhibit good AChE inhibition with IC50 of 11.42 to 22.21 µM. A molecular docking study has also been undertaken for compound 4n possessing the most potent AChE inhibitory activity, disclosing its binding to the peripheral anionic site of AChE enzymes.
Keywords: [3+2]-cycloaddition reaction; azomethine ylides; dispiropyrrolidine derivatives; succinimide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Kotha S., Panguluri N.R., Ali R. Design and Synthesis of Spirocycles. Eur. J. Org. Chem. 2017:5316–5342. doi: 10.1002/ejoc.201700439. - DOI
-
- El-Sharief A.M.S., Ammar Y.A., Belal A., El-Sharief M.A.M.S., Mohamed Y.A., Mehany A.B.M., Elhag Ali G.A.M., Ragab A. Design, Synthesis, Molecular Docking and Biological Activity Evaluation of Some Novel Indole Derivatives as Potent Anticancer Active Agents and Apoptosis Inducers. Bioorg. Chem. 2019;85:399–412. doi: 10.1016/j.bioorg.2019.01.016. - DOI - PubMed
-
- Lotfy G., El Sayed H., Said M.M., Aziz Y.M.A., Al-Dhfyan A., Al-Majid A.M., Barakat A. Regio- and Stereoselective Synthesis of New Spirooxindoles via 1,3-Dipolar Cycloaddition Reaction: Anticancer and Molecular Docking Studies. J. Photochem. Photobiol. B Biol. 2018;180:98–108. doi: 10.1016/j.jphotobiol.2018.01.026. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

