Phosphorylation of the overlooked tyrosine 310 regulates the structure, aggregation, and microtubule- and lipid-binding properties of Tau
- PMID: 32341125
- PMCID: PMC7278352
- DOI: 10.1074/jbc.RA119.012517
Phosphorylation of the overlooked tyrosine 310 regulates the structure, aggregation, and microtubule- and lipid-binding properties of Tau
Abstract
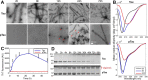
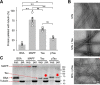
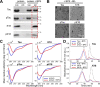
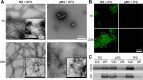
The microtubule-associated protein Tau is implicated in the pathogenesis of several neurodegenerative disorders, including Alzheimer's disease. Increasing evidence suggests that post-translational modifications play critical roles in regulating Tau's normal functions and its pathogenic properties in tauopathies. Very little is known about how phosphorylation of tyrosine residues influences the structure, aggregation, and microtubule- and lipid-binding properties of Tau. Here, we sought to determine the relative contributions of phosphorylation of one or several of the five tyrosine residues in Tau (Tyr-18, -29, -197, -310, and -394) to the regulation of its biophysical, aggregation, and functional properties. We used a combination of site-specific mutagenesis and in vitro phosphorylation by c-Abl kinase to generate Tau species phosphorylated at all five tyrosine residues, all tyrosine residues except Tyr-310 or Tyr-394 (pTau-Y310F and pTau-Y394F, respectively) and Tau phosphorylated only at Tyr-310 or Tyr-394 (4F/pTyr-310 or 4F/pTyr-394). We observed that phosphorylation of all five tyrosine residues, multiple N-terminal tyrosine residues (Tyr-18, -29, and -197), or specific phosphorylation only at residue Tyr-310 abolishes Tau aggregation and inhibits its microtubule- and lipid-binding properties. NMR experiments indicated that these effects are mediated by a local decrease in β-sheet propensity of Tau's PHF6 domain. Our findings underscore Tyr-310 phosphorylation has a unique role in the regulation of Tau aggregation, microtubule, and lipid interactions. These results also highlight the importance of conducting further studies to elucidate the role of Tyr-310 in the regulation of Tau's normal functions and pathogenic properties.
Keywords: Tau protein (Tau); aggregation; amyloid; lipid binding; microtubule; neurodegenerative disease; phosphorylation; phosphotyrosine; post-translational modification (PTM); tauopathy.
© 2020 Ait-Bouziad et al.
Conflict of interest statement
Conflict of interest—Prof. Hilal Lashuel is the founder and chief scientific officer of ND BioSciences SA.
Figures






References
-
- Delacourte A., and Défossez A. (1986) Biochemical characterization of an immune serum which specifically marks neurons in neurofibrillary degeneration in Alzheimer's disease. C. R. Acad. Sci. III 303, 439–444 - PubMed
-
- Arima K., Nakamura M., Sunohara N., Nishio T., Ogawa M., Hirai S., Kawai M., and Ikeda K. (1999) Immunohistochemical and ultrastructural characterization of neuritic clusters around ghost tangles in the hippocampal formation in progressive supranuclear palsy brains. Acta Neuropathol. 97, 565–576 10.1007/s004010051032 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

