Cytokines regulate the antigen-presenting characteristics of human circulating and tissue-resident intestinal ILCs
- PMID: 32341343
- PMCID: PMC7184749
- DOI: 10.1038/s41467-020-15695-x
Cytokines regulate the antigen-presenting characteristics of human circulating and tissue-resident intestinal ILCs
Abstract
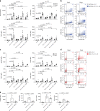
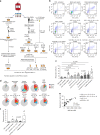
ILCs and T helper cells have been shown to exert bi-directional regulation in mice. However, how crosstalk between ILCs and CD4+ T cells influences immune function in humans is unknown. Here we show that human intestinal ILCs co-localize with T cells in healthy and colorectal cancer tissue and display elevated HLA-DR expression in tumor and tumor-adjacent areas. Although mostly lacking co-stimulatory molecules ex vivo, intestinal and peripheral blood (PB) ILCs acquire antigen-presenting characteristics triggered by inflammasome-associated cytokines IL-1β and IL-18. IL-1β drives the expression of HLA-DR and co-stimulatory molecules on PB ILCs in an NF-κB-dependent manner, priming them as efficient inducers of cytomegalovirus-specific memory CD4+ T-cell responses. This effect is strongly inhibited by the anti-inflammatory cytokine TGF-β. Our results suggest that circulating and tissue-resident ILCs have the intrinsic capacity to respond to the immediate cytokine milieu and regulate local CD4+ T-cell responses, with potential implications for anti-tumor immunity and inflammation.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

