Innate Immune Functions of Astrocytes are Dependent Upon Tumor Necrosis Factor-Alpha
- PMID: 32341377
- PMCID: PMC7184618
- DOI: 10.1038/s41598-020-63766-2
Innate Immune Functions of Astrocytes are Dependent Upon Tumor Necrosis Factor-Alpha
Abstract
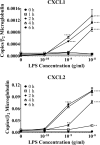
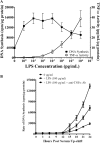
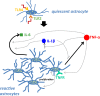
Acute inflammation is a key feature of innate immunity that initiates clearance and repair in infected or damaged tissues. Alternatively, chronic inflammation is implicated in numerous disease processes. The contribution of neuroinflammation to the pathogenesis of neurological conditions, including infection, traumatic brain injury, and neurodegenerative diseases, has become increasingly evident. Potential drivers of such neuroinflammation include toll-like receptors (TLRs). TLRs confer a wide array of functions on different cell types in the central nervous system (CNS). Importantly, how TLR activation affects astrocyte functioning is unclear. In the present study, we examined the role of TLR2/4 signaling on various astrocyte functions (i.e., proliferation, pro-inflammatory mediator production, regulatory mechanisms, etc) by stimulating astrocytes with potent exogenous TLR2/4 agonist, bacterial lipopolysaccharide (LPS). Newborn astrocytes were derived from WT, Tnfα-/-, Il1α-/-/Il1β-/-, and Tlr2-/-/Tlr4-/- mice as well as Sprague Dawley rats for all in vitro studies. LPS activated mRNA expression of different pro-inflammatory cytokines and chemokines in time- and concentration-dependent manners, and upregulated the proliferation of astrocytes based on increased 3H-thymidine update. Following LPS-mediated TLR2/4 activation, TNF-α and IL-1β self-regulated and modulated the expression of pro-inflammatory cytokines and chemokines. Polyclonal antibodies against TNF-α suppressed TLR2/4-mediated upregulation of astrocyte proliferation, supporting an autocrine/paracrine role of TNF-α on astrocyte proliferation. Astrocytes perform classical innate immune functions, which contradict the current paradigm that microglia are the main immune effector cells of the CNS. TNF-α plays a pivotal role in the LPS-upregulated astrocyte activation and proliferation, supporting their critical roles in in CNS pathogenesis.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

