A year-long extended release nanoformulated cabotegravir prodrug
- PMID: 32341511
- PMCID: PMC7384935
- DOI: 10.1038/s41563-020-0674-z
A year-long extended release nanoformulated cabotegravir prodrug
Abstract
Long-acting cabotegravir (CAB) extends antiretroviral drug administration from daily to monthly. However, dosing volumes, injection site reactions and health-care oversight are obstacles towards a broad usage. The creation of poloxamer-coated hydrophobic and lipophilic CAB prodrugs with controlled hydrolysis and tissue penetrance can overcome these obstacles. To such ends, fatty acid ester CAB nanocrystal prodrugs with 14, 18 and 22 added carbon chains were encased in biocompatible surfactants named NMCAB, NM2CAB and NM3CAB and tested for drug release, activation, cytotoxicity, antiretroviral activities, pharmacokinetics and biodistribution. Pharmacokinetics studies, performed in mice and rhesus macaques, with the lead 18-carbon ester chain NM2CAB, showed plasma CAB levels above the protein-adjusted 90% inhibitory concentration for up to a year. NM2CAB, compared with NMCAB and NM3CAB, demonstrated a prolonged drug release, plasma circulation time and tissue drug concentrations after a single 45 mg per kg body weight intramuscular injection. These prodrug modifications could substantially improve CAB's effectiveness.
Conflict of interest statement
Competing interests
B.J.E. and H.E.G. are named inventors on patents that cover the medicinal and polymer chemistry technologies employed in this manuscript encompassing the synthesis of long acting cabotegravir prodrugs and formulation manufacturing. H.E.G. is the Interim Director of the Nebraska Nanomedicine Production Plant, a good manufacturing program facility. The authors declare that this work was produced solely by the authors and that no other individuals or entities influenced any aspects of the work including, but not limited to, the study conception and design, data acquisition, analyses and interpretation, and writing of the manuscript. No other entities provided funds for the work. The authors further declare that they have received no financial compensation from any other third parties for any aspects of the published work. The remaining authors declare no competing interests.
Data availability
The data supporting the study’s findings are available within the article and its supplementary files or from the corresponding authors upon request. All the relevant data used to generate Figures 4a, 4c–k, 5a–h, 5i, 6a and 6b–c are included as Source Data.
Figures
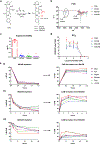




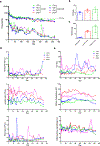
Comment in
-
Long-acting antiretroviral therapy.Nat Mater. 2020 Aug;19(8):826-827. doi: 10.1038/s41563-020-0731-7. Nat Mater. 2020. PMID: 32704135 No abstract available.
References
-
- Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV Epidemic: A Plan for the United States. JAMA 2019, 321(9): 844–845. - PubMed
-
- Currier JS. Monthly Injectable Antiretroviral Therapy - Version 1.0 of a New Treatment Approach. N Engl J Med 2020. - PubMed
-
- Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, Eron JJ, Yazdanpanah Y, Podzamczer D, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet 2017, 390(10101): 1499–1510. - PubMed
-
- Orkin C, Arasteh K, Hernandez-Mora MG, Pokrovsky V, Overton ET, Girard PM, et al. Long-Acting Cabotegravir and Rilpivirine after Oral Induction for HIV-1 Infection. N Engl J Med 2020. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS036126/NS/NINDS NIH HHS/United States
- R01 AI145542/AI/NIAID NIH HHS/United States
- P01 DA028555/DA/NIDA NIH HHS/United States
- P30 MH062261/MH/NIMH NIH HHS/United States
- R56 AI138613/AI/NIAID NIH HHS/United States
- R01 MH121402/MH/NIMH NIH HHS/United States
- P01 MH064570/MH/NIMH NIH HHS/United States
- R01 AG043540/AG/NIA NIH HHS/United States
- R01 NS034239/NS/NINDS NIH HHS/United States
- R01 MH115860/MH/NIMH NIH HHS/United States
- P01 NS043985/NS/NINDS NIH HHS/United States
- R01 AI158160/AI/NIAID NIH HHS/United States
- P01 NS031492/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

