Reconstitution of translesion synthesis reveals a mechanism of eukaryotic DNA replication restart
- PMID: 32341533
- PMCID: PMC7116498
- DOI: 10.1038/s41594-020-0418-4
Reconstitution of translesion synthesis reveals a mechanism of eukaryotic DNA replication restart
Abstract
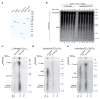
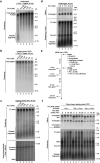
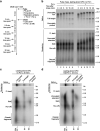
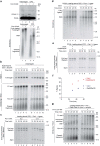
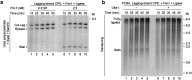
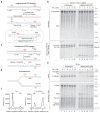
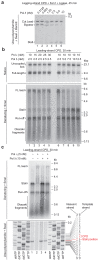
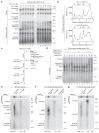
Leading-strand template aberrations cause helicase-polymerase uncoupling and impede replication fork progression, but the details of how uncoupled forks are restarted remain uncertain. Using purified proteins from Saccharomyces cerevisiae, we have reconstituted translesion synthesis (TLS)-mediated restart of a eukaryotic replisome following collision with a cyclobutane pyrimidine dimer. We find that TLS functions 'on the fly' to promote resumption of rapid replication fork rates, despite lesion bypass occurring uncoupled from the Cdc45-MCM-GINS (CMG) helicase. Surprisingly, the main lagging-strand polymerase, Pol δ, binds the leading strand upon uncoupling and inhibits TLS. Pol δ is also crucial for efficient recoupling of leading-strand synthesis to CMG following lesion bypass. Proliferating cell nuclear antigen monoubiquitination positively regulates TLS to overcome Pol δ inhibition. We reveal that these mechanisms of negative and positive regulation also operate on the lagging strand. Our observations have implications for both fork restart and the division of labor during leading-strand synthesis generally.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

