Laser fluorescence spectroscopy in predicting the formation of a keloid scar: preliminary results and the role of lipopigments
- PMID: 32341844
- PMCID: PMC7173908
- DOI: 10.1364/BOE.386029
Laser fluorescence spectroscopy in predicting the formation of a keloid scar: preliminary results and the role of lipopigments
Abstract
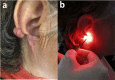
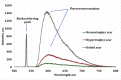
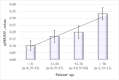
Keloid scars, in contrast to other scar types, significantly reduce the patient's quality of life. To develop a nondestructive optical diagnostic technique predicting the keloid scars formation in vivo, laser-induced fluorescence spectroscopy (LFS) was used to study the autofluorescence in skin of patients with various types of head and neck cicatricial deformities. The unexpected results were obtained for the endogenous fluorescence of lipofuscin. Significantly reduced autofluorescence of lipofuscin was registered both in the intact and in the keloid scar tissues in comparison with the intact and scar tissues in patients with hypertrophic and normotrophic scars. Sensitivity and specificity achieved by LFS in keloid diagnosis are 81.8% and 93.9% respectively. It could take place due to the changes in the reductive-oxidative balance in cells, as well as due to the proteolysis processes violation. Therefore, we suppose that the evaluation of the lipofuscin autofluorescence in skin before any surgical intervention could predict the probability of the subsequent keloid scars formation.
© 2020 Optical Society of America under the terms of the OSA Open Access Publishing Agreement.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Utsuki S., Oka H., Sato S., Suzuki S., Shimizu S., Tanaka S., Fujii K., “Possibility of using laser spectroscopy for the intraoperative detection of nonfluorescing brain tumors and the boundaries of brain tumor infiltrates,” J. Neurosurg. 104(4), 618–620 (2006).10.3171/jns.2006.104.4.618 - DOI - PubMed
-
- Van Dam G. M., Themelis G., Crane L. M., Harlaar N. J., Pleijhuis R. G., Kelder W., Sarantopoulos A., de Jong J. S., Arts H. J. G., van der Zee A. G. J., Bart J., Low P. S., Ntziachristos V., “Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: first in-human results,” Nat. Med. 17(10), 1315–1319 (2011).10.1038/nm.2472 - DOI - PubMed
-
- Bachmann L., Zezell D. M., Ribeiro A. D. C., Gomes L., Ito A. S., “Fluorescence spectroscopy of biological tissues—A review,” Appl. Spectrosc. Rev. 41(6), 575–590 (2006).10.1080/05704920600929498 - DOI
-
- Lakowicz J. R., Principles of Fluorescence Spectroscopy (Springer Science & Business Media, 2013).
-
- Georgakoudi I., Jacobson B. C., Müller M. G., Sheets E. E., Badizadegan K., Carr-Locke D. L., Crum C. P., Boone C. W., Dasari R. R., Van Dam J., Feld M. S., “NAD (P) H and collagen as in vivo quantitative fluorescent biomarkers of epithelial precancerous changes,” Cancer Res. 62(3), 682–687 (2002). - PubMed
LinkOut - more resources
Full Text Sources
