Paediatric drugs trials in China
- PMID: 32342015
- PMCID: PMC7173953
- DOI: 10.1136/bmjpo-2019-000618
Paediatric drugs trials in China
Abstract
Objective: Clinical trials of children's drugs are of great significance to rational drug use in children. However, paediatric drugs trials in China are facing complex challenges. At present, the investigation data on registration status of paediatric drug trials in China are still relatively lacking, and relevant research is urgently needed.
Methods: The advanced retrieval function is used to retrieve clinical trials data in the Clinical Trial.gov and Chinese Clinical Trial Registry databases in 22 April 2019. Fifteen key items were analysed to describe trial characteristics, including: registration number, study start date (year), mode of funding, type of disease, medicine type, research stage, research design, sample size, number of experimental groups, placebo group, blind method, implementation centre, child specific, newborn specific and participant age.
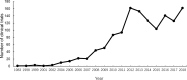
Results: A total of 1388 clinical trials of paediatric drugs conducted in China were registered. The number of paediatric drug trials grew steadily over time, from less than 20 per year before 2005 to more than 100 per year after 2012. Most clinical trials were postmarketing (n=800, 57.6%), single-centre (n=1045, 75.3%), intervention studies (n=1161, 83.6%) without blinded methods (1169, 84.2%) and funded by non-profit organisations (n=838, 60.4%). The number of clinical trials for antineoplastic agents (n=254, 18.3%), anti-infectives (n=156, 11.2%) and vaccines (n=154, 11.1%) is the largest.
Conclusion: Paediatric drug trials in China made a significant progress in recent years. Innovative method and trial design optimisation should be encouraged to accelerate paediatric clinical research. Pharmaceutical companies need to be further stimulated to carry out more high-quality paediatric clinical trials with support of paediatric drug legislation.
Keywords: evidence based medicine; paediatric practice.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

Similar articles
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Chinese Clinical Trial Registry 13-year data collection and analysis: geographic distribution, financial support, research phase, duration, and disease categories.Front Med (Lausanne). 2023 Oct 12;10:1203346. doi: 10.3389/fmed.2023.1203346. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37901406 Free PMC article.
-
Inhaled corticosteroids in children with persistent asthma: effects on growth.Evid Based Child Health. 2014 Dec;9(4):829-930. doi: 10.1002/ebch.1988. Evid Based Child Health. 2014. PMID: 25504972
-
Changes in Clinical Trials of Dermatological Drugs in Mainland China Between 2016 and 2022: A Narrative Review.Ther Innov Regul Sci. 2025 May;59(3):450-461. doi: 10.1007/s43441-025-00743-9. Epub 2025 Feb 13. Ther Innov Regul Sci. 2025. PMID: 39948234 Review.
-
The U.S. pediatric cancer clinical trials programmes: international implications and the way forward.Eur J Cancer. 1997 Aug;33(9):1439-47. doi: 10.1016/s0959-8049(97)00249-9. Eur J Cancer. 1997. PMID: 9337687 Review.
Cited by
-
Regulatory considerations for paediatric drug evaluation in China.BMJ Paediatr Open. 2023 May;7(1):e001666. doi: 10.1136/bmjpo-2022-001666. BMJ Paediatr Open. 2023. PMID: 37130653 Free PMC article. Review.
-
Pediatric Clinical Trials in Mainland China Over the Past Decade (From 2009 to 2020).Front Med (Lausanne). 2021 Oct 4;8:745676. doi: 10.3389/fmed.2021.745676. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34671625 Free PMC article.
-
Current situation of pediatric clinical trials in China: focus on trials for drug marketing application and administrative approval.BMC Pediatr. 2022 Mar 18;22(1):144. doi: 10.1186/s12887-022-03208-2. BMC Pediatr. 2022. PMID: 35303815 Free PMC article.
References
-
- Gore R, Chugh P, Tripathi C, et al. . Pediatric off-label and unlicensed drug use and its implications 2017;12:18–25. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
