Growth Hormone Stops Excessive Inflammation After Partial Hepatectomy, Allowing Liver Regeneration and Survival Through Induction of H2-Bl/HLA-G
- PMID: 32342533
- PMCID: PMC7894545
- DOI: 10.1002/hep.31297
Growth Hormone Stops Excessive Inflammation After Partial Hepatectomy, Allowing Liver Regeneration and Survival Through Induction of H2-Bl/HLA-G
Abstract
Background and aims: Growth hormone (GH) is important for liver regeneration after partial hepatectomy (PHx). We investigated this process in C57BL/6 mice that express different forms of the GH receptor (GHR) with deletions in key signaling domains.
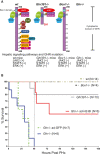
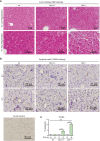
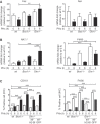
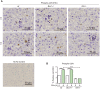
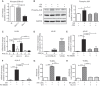
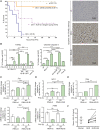
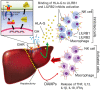
Approach and results: PHx was performed on C57BL/6 mice lacking GHR (Ghr-/- ), disabled for all GH-dependent Janus kinase 2 signaling (Box1-/- ), or lacking only GH-dependent signal transducer and activator of transcription 5 (STAT5) signaling (Ghr391-/- ), and wild-type littermates. C57BL/6 Ghr-/- mice showed striking mortality within 48 hours after PHx, whereas Box1-/- or Ghr391-/- mice survived with normal liver regeneration. Ghr-/- mortality was associated with increased apoptosis and elevated natural killer/natural killer T cell and macrophage cell markers. We identified H2-Bl, a key immunotolerance protein, which is up-regulated by PHx through a GH-mediated, Janus kinase 2-independent, SRC family kinase-dependent pathway. GH treatment was confirmed to up-regulate expression of the human homolog of H2-Bl (human leukocyte antigen G [HLA-G]) in primary human hepatocytes and in the serum of GH-deficient patients. We find that injury-associated innate immune attack by natural killer/natural killer T cell and macrophage cells are instrumental in the failure of liver regeneration, and this can be overcome in Ghr-/- mice by adenoviral delivery of H2-Bl or by infusion of HLA-G protein. Further, H2-Bl knockdown in wild-type C57BL/6 mice showed elevated markers of inflammation after PHx, whereas Ghr-/- backcrossed on a strain with high endogenous H2-Bl expression showed a high rate of survival following PHx.
Conclusions: GH induction of H2-Bl expression is crucial for reducing innate immune-mediated apoptosis and promoting survival after PHx in C57BL/6 mice. Treatment with HLA-G may lead to improved clinical outcomes following liver surgery or transplantation.
© 2020 The Authors. Hepatology published by Wiley Periodicals LLC on behalf of American Association for the Study of Liver Diseases.
Figures

Comment in
-
Reply.Hepatology. 2021 Mar;73(3):1239. doi: 10.1002/hep.31531. Hepatology. 2021. PMID: 32865232 No abstract available.
-
Letter to the Editor: Growth Hormone Stops Excessive Inflammation After Partial Hepatectomy, Allowing Liver Regeneration and Survival by Induction of H2-Bl/HLA-G.Hepatology. 2021 Mar;73(3):1238. doi: 10.1002/hep.31532. Epub 2021 Feb 26. Hepatology. 2021. PMID: 32865250 No abstract available.
References
-
- Notas G, Kisseleva T, Brenner D. NK and NKT cells in liver injury and fibrosis. Clin Immunol 2009;130:16‐26. - PubMed
-
- Pennisi PA, Kopchick JJ, Thorgeirsson S, LeRoith D, Yakar S. Role of growth hormone (GH) in liver regeneration. Endocrinology 2004;145:4748‐4755. - PubMed
-
- Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, et al. Liver failure and defective hepatocyte regeneration in interleukin‐6‐deficient mice. Science 1996;274:1379‐1383. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

