Dengue Infection in Children in Fortaleza, Brazil: A 3-Year School-Based Prospective Cohort Study
- PMID: 32342838
- PMCID: PMC7356456
- DOI: 10.4269/ajtmh.19-0521
Dengue Infection in Children in Fortaleza, Brazil: A 3-Year School-Based Prospective Cohort Study
Abstract
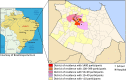
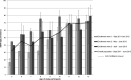
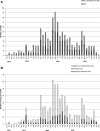
Dengue is endemic in Brazil. The dengue surveillance system's reliance on passive reporting may underestimate disease incidence and cannot detect asymptomatic/pauci-symptomatic cases. In this 3-year prospective cohort study (NCT01391819) in 5- to 13-year-old children from nine schools in Fortaleza (N = 2,117), we assessed dengue virus (DENV) infection seroprevalence by IgG indirect ELISA at yearly visits and disease incidence through active and enhanced passive surveillance. Real-time quantitative polymerase chain reaction (RT-qPCR) and DENV IgM/IgG capture ELISA were used for diagnosis. We further characterized confirmed and probable cases with a plaque reduction neutralization test. At enrollment, 54.1% (95% CI: 46.6, 61.4) of children were DENV IgG positive. The annual incidence of laboratory-confirmed symptomatic dengue cases was 11.0 (95% CI: 7.3, 14.7), 18.1 (10.4, 25.7), and 10.2 (0.7, 19.7), and of laboratory-confirmed or probable dengue cases with neutralizing antibody profile evocative of dengue exposure was 13.2 (6.6, 19.9), 18.7 (5.3, 32.2), and 8.4 (2.4, 19.2) per 1,000 child-years in 2012, 2013, and 2014, respectively. By RT-qPCR, we identified 14 DENV-4 cases in 2012-2013 and seven DENV-1 cases in 2014. During the course of the study, 32.8% of dengue-naive children experienced a primary infection. Primary inapparent dengue infection was detected in 20.3% (95% CI: 13.6, 29.1) of dengue-naive children in 2012, 8.7% (6.9, 10.9) in 2013, and 5.1% (4.4, 6.0) in 2014. Our results confirmed the high dengue endemicity in Fortaleza, with active and enhanced passive surveillance detecting three to five times more cases than the National System of Disease Notification.
Conflict of interest statement
Disclosure: F. H., C. M., and A. G. are employees of the GSK group of companies. A.G. hold shares in the GSK group of companies and I.C.B.C., J.K.B.C., Z.C.B.C., F.M.C.A., W.D.S., A.C.D., and B.B. declare no financial and non-financial relationships and activities and no conflicts of interest. Dengvaxia is a trademark of Sanofi Pasteur. True Blue is a trademark of SeraCare Life Sciences Inc.
Figures

References
-
- Deen JL, Harris E, Wills B, Balmaseda A, Hammond SN, Rocha C, Dung NM, Hung NT, Hien TT, Farrar JJ, 2006. The WHO dengue classification and case definitions: time for a reassessment. Lancet 368: 170–173. - PubMed
-
- PAHO , 2011–2017. Dengue: Reported Cases 2010–2017. Available at: http://www.paho.org/hq/index.php?option=com_topics&view=rdmore&cid=6290&... Accessed June 7, 2018.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

