ADSC-Exos containing MALAT1 promotes wound healing by targeting miR-124 through activating Wnt/β-catenin pathway
- PMID: 32342982
- PMCID: PMC7214401
- DOI: 10.1042/BSR20192549
ADSC-Exos containing MALAT1 promotes wound healing by targeting miR-124 through activating Wnt/β-catenin pathway
Abstract
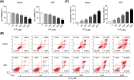
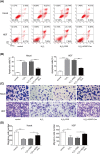
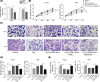
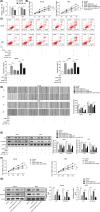
Cutaneous wound is a soft tissue injury that is difficult to heal during aging. It has been demonstrated that adipose-derived stem cells (ADSCs) and its secreted exosomes exert crucial functions in cutaneous wound healing. The present study aimed to elucidate the mechanism of exosomes derived from ADSCs (ADSC-Exos) containing MALAT1 in wound healing. ADSCs were isolated from human normal subcutaneous adipose tissues and identified by flow cytometry analysis. Exosomes were extracted from ADSC supernatants and MALAT1 expression was determined using qRT-PCR analysis. HaCaT and HDF cells were exposed to hydrogen peroxide (H2O2) for simulating the skin lesion model. Subsequently, CCK-8, flow cytometry, wound healing and transwell assays were employed to validate the role of ADSC-Exos containing MALAT1 in the skin lesion model. Besides, cells were transfected with sh-MALAT1 to verify the protective role of MALAT1 in wound healing. The binding relationship between MALAT1 and miR-124 were measured by dual-luciferase reporter assay. ADSC-Exos promoted cell proliferation, migration, and inhibited cell apoptosis of HaCaT and HDF cells impaired by H2O2. However, the depletion of MALAT1 in ADSC-Exos lose these protective effects on HaCaT and HDF cells. Moreover, miR-124 was identified to be a target of MALAT1. Furthermore, ADSC-Exos containing MALAT1 could mediate H2O2-induced wound healing by targeting miR-124 and activating Wnt/β-catenin pathway. ADSC-Exos containing MALAT1 play a positive role in cutaneous wound healing possibly via targeting miR-124 through activating the Wnt/β-catenin pathway, which may provide novel insights into the therapeutic target for cutaneous wound healing.
Keywords: MALAT1; adipose-derived stem cell; cutaneous wound healing; exosomes; miR-124.
© 2020 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures


References
-
- Lewis C.J., Mardaryev A.N., Poterlowicz K., Sharova T.Y., Aziz A., Sharpe D.T. et al. . (2014) Bone morphogenetic protein signaling suppresses wound-induced skin repair by inhibiting keratinocyte proliferation and migration. J. Invest. Dermatol. 134, 827–837 10.1038/jid.2013.419 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

