Disease-associated mutations in the human TRPM3 render the channel overactive via two distinct mechanisms
- PMID: 32343227
- PMCID: PMC7255801
- DOI: 10.7554/eLife.55634
Disease-associated mutations in the human TRPM3 render the channel overactive via two distinct mechanisms
Abstract
Transient Receptor Potential Melastatin 3 (TRPM3) is a Ca2+ permeable non-selective cation channel activated by heat and chemical agonists such as pregnenolone sulfate and CIM0216. TRPM3 mutations in humans were recently reported to be associated with intellectual disability and epilepsy; the functional effects of those mutations, however, were not reported. Here, we show that both disease-associated mutations in the human TRPM3 render the channel overactive, but likely via different mechanisms. The Val to Met substitution in the S4-S5 loop induced a larger increase in basal activity and agonist sensitivity at room temperature than the Pro to Gln substitution in the extracellular segment of S6. In contrast, heat activation was increased more by the S6 mutant than by the S4-S5 segment mutant. Both mutants were inhibited by the TRPM3 antagonist primidone, suggesting a potential therapeutic intervention to treat this disease.
Keywords: TRPM3; channelopathy; ion channel; molecular biophysics; neuroscience; none; structural biology.
Plain language summary
Inherited brain disorders often cause severe problems for those affected by them. One example is a group of diseases, collectively termed “developmental and epileptic encephalopathies”, or DEE for short. People with these diseases usually have both epilepsy and intellectual disabilities, and in some patients these conditions are associated with two mutations that change a gene called TRPM3. The TRPM3 gene encodes a protein called an ion channel. Ion channels form pores on the surfaces of cells. When channels are active, the pores open, allowing charged particles – which, in the case of TRPM3, are sodium and calcium ions – to pass through, carrying tiny electrical currents. In the nervous system, ion channels help nerve cells communicate and also allow them to sense changes in the environment. The TRPM3 channel is known to open in response to heat and certain chemical “activators”. In mice, TRPM3 is found in sensory nerve cells, where it acts as a heat sensor. Although altering TRPM3 in mice affects their ability to sense intense or painful heat stimuli, they are otherwise completely normal and have no symptoms resembling human DEE disorders. Although TRPM3 is found in the human brain, little is known about its role there or what effects the DEE-associated mutations have on its activity. Zhao et al. therefore set out to determine, whether each of the mutation was a ‘loss of function’, meaning that it stopped the channel from opening, or a ‘gain of function’, meaning it made the channel open more often. Frog egg cells and mammalian cells grown in the laboratory were engineered to produce the TRPM3 ion channel. Measurements of electrical activity on these cells revealed that the two mutations seen in people with DEE were both ‘gain of function’. Both mutants were more sensitive to heat and chemical activators than the normal protein. They were also more active overall, even without any stimuli. However, one mutation had a greater effect on heat sensitivity, while the other caused a larger increase in chemical-induced activity. Imaging experiments revealed that both mutant channels also increased the amount of calcium inside the cells. This could explain why the mutations cause disease, since abnormally high calcium levels can damage nerve cells. In addition, the epilepsy drug primidone switched off the mutant channels, pointing to potential treatment of this disease using primidone.
© 2020, Zhao et al.
Conflict of interest statement
SZ, YY, TR No competing interests declared
Figures


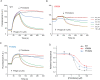

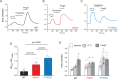
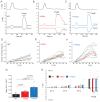
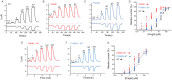



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

