Finding the Dose for Hydroxychloroquine Prophylaxis for COVID-19: The Desperate Search for Effectiveness
- PMID: 32344449
- PMCID: PMC7267462
- DOI: 10.1002/cpt.1874
Finding the Dose for Hydroxychloroquine Prophylaxis for COVID-19: The Desperate Search for Effectiveness
Abstract
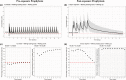
Hydroxychloroquine is an antimalarial drug being tested as a potential treatment for the novel coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2. Although the efficacy of hydroxychloroquine for COVID-19 remains uncertain, it may serve as a potential prophylactic agent especially in those at high risk, such as healthcare workers, household contacts of infected patients, and the immunocompromised. Our aim was to identify possible hydroxychloroquine dosing regimens through simulation in those at high risk of infections by optimizing exposures above the in vitro generated half maximal effective concentration (EC50 ) and to help guide researchers in dose-selection for COVID-19 prophylactic studies. To maintain weekly troughs above EC50 in > 50% of subjects at steady-state in a pre-exposure prophylaxis setting, an 800 mg loading dose followed by 400 mg twice or 3 times weekly is required. In an exposure driven, post-exposure prophylaxis setting, 800 mg loading dose followed in 6 hours by 600 mg, then 600 mg daily for 4 more days achieved daily troughs above EC50 in > 50% subjects. These doses are higher than recommended for malaria chemoprophylaxis, and clinical trials are needed to establish safety and efficacy.
© 2020 The Authors Clinical Pharmacology & Therapeutics © 2020 American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures
References
-
- Dong, L. , Hu, S. & Gao, J. Discovering drugs to treat coronavirus disease 2019 (COVID‐19). Drug. Discov. Ther. 14, 58–60 (2020). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

