HER2-Specific Pseudomonas Exotoxin A PE25 Based Fusions: Influence of Targeting Domain on Target Binding, Toxicity, and In Vivo Biodistribution
- PMID: 32344762
- PMCID: PMC7238247
- DOI: 10.3390/pharmaceutics12040391
HER2-Specific Pseudomonas Exotoxin A PE25 Based Fusions: Influence of Targeting Domain on Target Binding, Toxicity, and In Vivo Biodistribution
Abstract
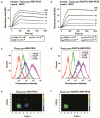
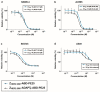
The human epidermal growth factor receptor 2 (HER2) is a clinically validated target for cancer therapy, and targeted therapies are often used in regimens for patients with a high HER2 expression level. Despite the success of current drugs, a number of patients succumb to their disease, which motivates development of novel drugs with other modes of action. We have previously shown that an albumin binding domain-derived affinity protein with specific affinity for HER2, ADAPT6, can be used to deliver the highly cytotoxic protein domain PE25, a derivative of Pseudomonas exotoxin A, to HER2 overexpressing malignant cells, leading to potent and specific cell killing. In this study we expanded the investigation for an optimal targeting domain and constructed two fusion toxins where a HER2-binding affibody molecule, ZHER2:2891, or the dual-HER2-binding hybrid ZHER2:2891-ADAPT6 were used for cancer cell targeting. We found that both targeting domains conferred strong binding to HER2; both to the purified extracellular domain and to the HER2 overexpressing cell line SKOV3. This resulted in fusion toxins with high cytotoxic potency toward cell lines with high expression levels of HER2, with EC50 values between 10 and 100 pM. For extension of the plasma half-life, an albumin binding domain was also included. Intravenous injection of the fusion toxins into mice showed a profound influence of the targeting domain on biodistribution. Compared to previous results, with ADAPT6 as targeting domain, ZHER2:2891 gave rise to further extension of the plasma half-life and also shifted the clearance route of the fusion toxin from the liver to the kidneys. Collectively, the results show that the targeting domain has a major impact on uptake of PE25-based fusion toxins in different organs. The results also show that PE25-based fusion toxins with high affinity to HER2 do not necessarily increase the cytotoxicity beyond a certain point in affinity. In conclusion, ZHER2:2891 has the most favorable characteristics as targeting domain for PE25.
Keywords: HER2; affibody molecule; cancer; half-life extension; pseudomonas exotoxin A.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Vogel C.L., Cobleigh M.A., Tripathy D., Gutheil J.C., Harris L.N., Fehrenbacher L., Slamon D.J., Murphy M., Novotny W.F., Burchmore M., et al. Efficacy and Safety of Trastuzumab as a Single Agent in First-Lin Treatment of HER2-Overexpressing Metastatic Breast Cancer. J. Clin. Oncol. 2003;20:719–726. doi: 10.1200/JCO.2002.20.3.719. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

