Investigating the effect of clinical pharmacist intervention in transitions of care on drug-related hospital readmissions among the elderly: study protocol for a randomised controlled trial
- PMID: 32345700
- PMCID: PMC7213854
- DOI: 10.1136/bmjopen-2019-036650
Investigating the effect of clinical pharmacist intervention in transitions of care on drug-related hospital readmissions among the elderly: study protocol for a randomised controlled trial
Abstract
Introduction: Drug-related problems (DRPs) are a major cause of unplanned hospital admissions among elderly people, and transitions of care have been emphasised as a key area for improving patient safety. We have designed a complex clinical pharmacist intervention that targets people ≥75 years of age undergoing transitions of care from hospital to home and primary care. The main objective is to investigate if the intervention can reduce the risk of unplanned drug-related readmission within the first 180 days after the person is discharged from hospital.
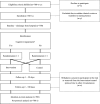
Methods and analysis: This is a randomised, controlled, superiority trial with two parallel arms. A total of 700 people ≥75 years will be assigned to either intervention or routine care (control). The intervention, which aims to find and manage DRPs, is initiated within a week of the person being discharged from hospital and combines repeated medical chart reviews, phone interviews and in some cases medication reviews. People in both study arms may have been the subject of a medication review during their ward stay. As the primary outcome, we will measure time until unplanned drug-related readmission within 180 days of leaving hospital and use log rank tests and Cox proportional hazard models to analyse differences between the groups. Further investigations of subgroup effects and adjustments of the regression models will be based on heart failure and cognitive impairment as prognostic factors.
Ethics and dissemination: The study has been approved by the Regional Ethical Review Board in Umeå (registration numbers 2017-69-31M, 2018-83-32M and 2018-254-32M). We intend to publish the results with open access in international peer-reviewed journals and present our findings at international conferences. The trial is expected to result in more than one published article and form part of two PhD theses.
Trial registration number: NCT03671629.
Keywords: clinical pharmacology; clinical trials; geriatric medicine.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
