Investigating APOE, APP-Aβ metabolism genes and Alzheimer's disease GWAS hits in brain small vessel ischemic disease
- PMID: 32345996
- PMCID: PMC7188838
- DOI: 10.1038/s41598-020-63183-5
Investigating APOE, APP-Aβ metabolism genes and Alzheimer's disease GWAS hits in brain small vessel ischemic disease
Abstract
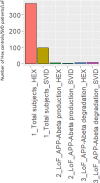
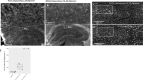
Alzheimer's disease and small vessel ischemic disease frequently co-exist in the aging brain. However, pathogenic links between these 2 disorders are yet to be identified. Therefore we used Taqman genotyping, exome and RNA sequencing to investigate Alzheimer's disease known pathogenic variants and pathways: APOE ε4 allele, APP-Aβ metabolism and late-onset Alzheimer's disease main genome-wide association loci (APOE, BIN1, CD33, MS4A6A, CD2AP, PICALM, CLU, CR1, EPHA1, ABCA7) in 96 early-onset small vessel ischemic disease Caucasian patients and 368 elderly neuropathologically proven controls (HEX database) and in a mouse model of cerebral hypoperfusion. Only a minority of patients (29%) carried APOE ε4 allele. We did not detect any pathogenic mutation in APP, PSEN1 and PSEN2 and report a burden of truncating mutations in APP-Aß degradation genes. The single-variant association test identified 3 common variants with a likely protective effect on small vessel ischemic disease (0.54>OR > 0.32, adj. p-value <0.05) (EPHA1 p.M900V and p.V160A and CD33 p.A14V). Moreover, 5/17 APP-Aß catabolism genes were significantly upregulated (LogFC > 1, adj. p-val<0.05) together with Apoe, Ms4a cluster and Cd33 during brain hypoperfusion and their overexpression correlated with the ischemic lesion size. Finally, the detection of Aβ oligomers in the hypoperfused hippocampus supported the link between brain ischemia and Alzheimer's disease pathology.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

