Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study
- PMID: 32346491
- PMCID: PMC7185795
- DOI: 10.1016/j.eng.2020.03.007
Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study
Abstract
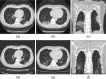
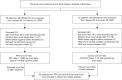
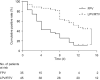
There is currently an outbreak of respiratory disease caused by a novel coronavirus. The virus has been named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the disease it causes has been named coronavirus disease 2019 (COVID-19). More than 16% of patients developed acute respiratory distress syndrome, and the fatality ratio was 1%-2%. No specific treatment has been reported. Herein, we examined the effects of favipiravir (FPV) versus lopinavir (LPV)/ritonavir (RTV) for the treatment of COVID-19. Patients with laboratory-confirmed COVID-19 who received oral FPV (Day 1: 1600 mg twice daily; Days 2-14: 600 mg twice daily) plus interferon (IFN)-α by aerosol inhalation (5 million international unit (IU) twice daily) were included in the FPV arm of this study, whereas patients who were treated with LPV/RTV (Days 1-14: 400 mg/100 mg twice daily) plus IFN-α by aerosol inhalation (5 million IU twice daily) were included in the control arm. Changes in chest computed tomography (CT), viral clearance, and drug safety were compared between the two groups. For the 35 patients enrolled in the FPV arm and the 45 patients in the control arm, all baseline characteristics were comparable between the two arms. A shorter viral clearance median time was found for the FPV arm versus the control arm (4 d (interquartile range (IQR): 2.5-9) versus 11 d (IQR: 8-13), P < 0.001). The FPV arm also showed significant improvement in chest CT compared with the control arm, with an improvement rate of 91.43% versus 62.22% (P = 0.004). After adjustment for potential confounders, the FPV arm also showed a significantly higher improvement rate in chest CT. Multivariable Cox regression showed that FPV was independently associated with faster viral clearance. In addition, fewer adverse events were found in the FPV arm than in the control arm. In this open-label before-after controlled study, FPV showed better therapeutic responses on COVID-19 in terms of disease progression and viral clearance. These preliminary clinical results provide useful information of treatments for SARS-CoV-2 infection.
Keywords: Antiviral therapy; COVID-19; Favipiravir; Open-label nonrandomized control study; SARS-CoV-2.
© 2020 THE AUTHORS.
Figures
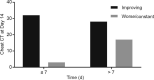
References
-
- National Health Commission of the People’s Republic of China. Daily briefing on novel coronavirus cases in China [Internet]. Beijing: National Health Commission of the People’s Republic of China; c2020 [updated 2020 Mar 12; cited 2020; c2020 Feb 25]. Available form: http://en.nhc.gov.cn/DailyBriefing.html.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
