The Renal Physiology of Pendrin-Positive Intercalated Cells
- PMID: 32347156
- PMCID: PMC7474261
- DOI: 10.1152/physrev.00011.2019
The Renal Physiology of Pendrin-Positive Intercalated Cells
Abstract
Intercalated cells (ICs) are found in the connecting tubule and the collecting duct. Of the three IC subtypes identified, type B intercalated cells are one of the best characterized and known to mediate Cl- absorption and HCO3- secretion, largely through the anion exchanger pendrin. This exchanger is thought to act in tandem with the Na+-dependent Cl-/HCO3- exchanger, NDCBE, to mediate net NaCl absorption. Pendrin is stimulated by angiotensin II and aldosterone administration via the angiotensin type 1a and the mineralocorticoid receptors, respectively. It is also stimulated in models of metabolic alkalosis, such as with NaHCO3 administration. In some rodent models, pendrin-mediated HCO3- secretion modulates acid-base balance. However, of probably more physiological or clinical significance is the role of these pendrin-positive ICs in blood pressure regulation, which occurs, at least in part, through pendrin-mediated renal Cl- absorption, as well as their effect on the epithelial Na+ channel, ENaC. Aldosterone stimulates ENaC directly through principal cell mineralocorticoid hormone receptor (ligand) binding and also indirectly through its effect on pendrin expression and function. In so doing, pendrin contributes to the aldosterone pressor response. Pendrin may also modulate blood pressure in part through its action in the adrenal medulla, where it modulates the release of catecholamines, or through an indirect effect on vascular contractile force. In addition to its role in Na+ and Cl- balance, pendrin affects the balance of other ions, such as K+ and I-. This review describes how aldosterone and angiotensin II-induced signaling regulate pendrin and the contribution of pendrin-positive ICs in the kidney to distal nephron function and blood pressure.
Keywords: Cl−/HCO3− exchange; ENaC; Slc26a4; blood pressure; intercalated cells; pendrin.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



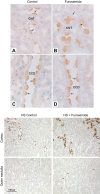

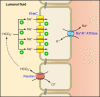

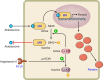


References
-
- Ackermann D, Gresko N, Carrel M, Loffing-Cueni D, Habermehl D, Gomez-Sanchez C, Rossier BC, Loffing J. In vivo nuclear translocation of mineralocorticoid and glucocorticoid receptors in rat kidney: differential effect of corticosteroids along the distal tubule. Am J Physiol Renal Physiol 299: F1473–F1485, 2010. doi:10.1152/ajprenal.00437.2010. - DOI - PubMed
-
- Amlal H, Petrovic S, Xu J, Wang Z, Sun X, Barone S, Soleimani M. Deletion of the anion exchanger Slc26a4 (pendrin) decreases apical Cl−/ exchanger activity and impairs bicarbonate secretion in kidney collecting duct. Am J Physiol Cell Physiol 299: C33–C41, 2010. doi:10.1152/ajpcell.00033.2010. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

