Effect of Chloroquine, Hydroxychloroquine, and Azithromycin on the Corrected QT Interval in Patients With SARS-CoV-2 Infection
- PMID: 32347743
- PMCID: PMC7299095
- DOI: 10.1161/CIRCEP.120.008662
Effect of Chloroquine, Hydroxychloroquine, and Azithromycin on the Corrected QT Interval in Patients With SARS-CoV-2 Infection
Abstract
Background: The novel SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) is responsible for the global coronavirus disease 2019 pandemic. Small studies have shown a potential benefit of chloroquine/hydroxychloroquine±azithromycin for the treatment of coronavirus disease 2019. Use of these medications alone, or in combination, can lead to a prolongation of the QT interval, possibly increasing the risk of Torsade de pointes and sudden cardiac death.
Methods: Hospitalized patients treated with chloroquine/hydroxychloroquine±azithromycin from March 1 to the 23 at 3 hospitals within the Northwell Health system were included in this prospective, observational study. Serial assessments of the QT interval were performed. The primary outcome was QT prolongation resulting in Torsade de pointes. Secondary outcomes included QT prolongation, the need to prematurely discontinue any of the medications due to QT prolongation, and arrhythmogenic death.
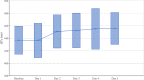
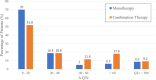
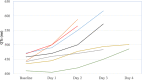
Results: Two hundred one patients were treated for coronavirus disease 2019 with chloroquine/hydroxychloroquine. Ten patients (5.0%) received chloroquine, 191 (95.0%) received hydroxychloroquine, and 119 (59.2%) also received azithromycin. The primary outcome of torsade de pointes was not observed in the entire population. Baseline corrected QT interval intervals did not differ between patients treated with chloroquine/hydroxychloroquine (monotherapy group) versus those treated with combination group (chloroquine/hydroxychloroquine and azithromycin; 440.6±24.9 versus 439.9±24.7 ms, P=0.834). The maximum corrected QT interval during treatment was significantly longer in the combination group versus the monotherapy group (470.4±45.0 ms versus 453.3±37.0 ms, P=0.004). Seven patients (3.5%) required discontinuation of these medications due to corrected QT interval prolongation. No arrhythmogenic deaths were reported.
Conclusions: In the largest reported cohort of coronavirus disease 2019 patients to date treated with chloroquine/hydroxychloroquine±azithromycin, no instances of Torsade de pointes, or arrhythmogenic death were reported. Although use of these medications resulted in QT prolongation, clinicians seldomly needed to discontinue therapy. Further study of the need for QT interval monitoring is needed before final recommendations can be made.
Keywords: COVID-19; QT prolongation; azithromycin; chloroquine; hydroxychloroquine; pandemic.
Figures
References
-
- World Health Organization. Coronavirus Disease 2019 (COVID-19) Situation Report 74. Geneva, Switzerland: WHO; 2020.
-
- Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, Liu X, Zhao L, Dong E, Song C, et al. In Vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020:ciaa237. doi: 10.1093/cid/ciaa237. - PMC - PubMed
-
- Mitra RL, Greenstein SA, Epstein LM. An algorithm for managing QT prolongation in Coronavirus Disease 2019 (COVID-19) patients treated with either chloroquine or hydroxychloroquine in conjunction with azithromycin: possible benefits of intravenous lidocaine. HeartRhythm Case Reports. 2020;6:244–248. doi: 10.1016/j.hrcr.2020.03.016. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

