SF3B1-mutant MDS as a distinct disease subtype: a proposal from the International Working Group for the Prognosis of MDS
- PMID: 32347921
- PMCID: PMC7362582
- DOI: 10.1182/blood.2020004850
SF3B1-mutant MDS as a distinct disease subtype: a proposal from the International Working Group for the Prognosis of MDS
Erratum in
-
Malcovati L, Stevenson K, Papaemmanuil E, et al. SF3B1-mutant MDS as a distinct disease subtype: a proposal from the International Working Group for the Prognosis of MDS. Blood. 2020;136(2):157-170.Blood. 2021 May 27;137(21):3003. doi: 10.1182/blood.2021012067. Blood. 2021. PMID: 34042976 Free PMC article. No abstract available.
Abstract
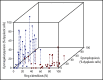
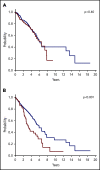
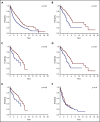
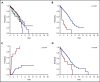
The 2016 revision of the World Health Organization classification of tumors of hematopoietic and lymphoid tissues is characterized by a closer integration of morphology and molecular genetics. Notwithstanding, the myelodysplastic syndrome (MDS) with isolated del(5q) remains so far the only MDS subtype defined by a genetic abnormality. Approximately half of MDS patients carry somatic mutations in spliceosome genes, with SF3B1 being the most commonly mutated one. SF3B1 mutation identifies a condition characterized by ring sideroblasts (RS), ineffective erythropoiesis, and indolent clinical course. A large body of evidence supports recognition of SF3B1-mutant MDS as a distinct nosologic entity. To further validate this notion, we interrogated the data set of the International Working Group for the Prognosis of MDS (IWG-PM). Based on the findings of our analyses, we propose the following diagnostic criteria for SF3B1-mutant MDS: (1) cytopenia as defined by standard hematologic values, (2) somatic SF3B1 mutation, (3) morphologic dysplasia (with or without RS), and (4) bone marrow blasts <5% and peripheral blood blasts <1%. Selected concomitant genetic lesions represent exclusion criteria for the proposed entity. In patients with clonal cytopenia of undetermined significance, SF3B1 mutation is almost invariably associated with subsequent development of overt MDS with RS, suggesting that this genetic lesion might provide presumptive evidence of MDS in the setting of persistent unexplained cytopenia. Diagnosis of SF3B1-mutant MDS has considerable clinical implications in terms of risk stratification and therapeutic decision making. In fact, this condition has a relatively good prognosis and may respond to luspatercept with abolishment of the transfusion requirement.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: R.B. received consulting fees from AbbVie, Astex, Celgene, Daiichi-Sankyo, Forty Seven, and NeoGenomics; honoraria for serving on steering and data safety monitoring committees for Celgene; and research funding from Celgene and Takeda. B.L.E. received research funding from Celgene and Deerfield; plays a consulting role from GRAIL; plays an advisory role and holds equity in Skyhawk Therapeutics and Exo Therapeutics. M.H. received honoraria from Novartis, Pfizer, and PriME Oncology; plays a consulting or advisory role for AbbVie, Bayer Pharma AG, Daiichi Sankyo, Novartis, and Pfizer; and received research funding (to institution) from Astellas, Bayer Pharma AG, BergenBio, Daiichi Sankyo, Karyopharm, Novartis, Pfizer, and Roche. M.R.S received research funding from Astex, Takeda, TG Therapeutics; Equity–Karyopharm; consulting fees for AbbVie, BMS, Celgene, Incyte, Karyopharm, Ryvu, Sierra Oncology, Takeda, TG Therapeutics. D.P.S. received institutional research funding from H3 Biosciences and consulting fees for Celgene. The remaining authors declare no competing financial interests.
Figures

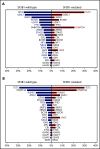
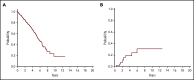
Comment in
-
SF3B1: the lord of the rings in MDS.Blood. 2020 Jul 9;136(2):149-151. doi: 10.1182/blood.2020005719. Blood. 2020. PMID: 32645165 No abstract available.
-
SF3B1-mutant CMML defines a predominantly dysplastic CMML subtype with a superior acute leukemia-free survival.Blood Adv. 2020 Nov 24;4(22):5716-5721. doi: 10.1182/bloodadvances.2020003345. Blood Adv. 2020. PMID: 33216886 Free PMC article. No abstract available.
References
-
- Swerdlow SH, Campo E, Harris NL, et al. , eds.. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed. Lyon, France: IARC; 2017.
-
- Cazzola M. Introduction to a review series: the 2016 revision of the WHO classification of tumors of hematopoietic and lymphoid tissues. Blood. 2016;127(20):2361-2364. - PubMed
-
- Arber DA, Orazi A, Hasserjian R, et al. . The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

