Pharmacokinetics, Pharmacodynamics, Safety, and Tolerability of Dupilumab in Healthy Adult Subjects
- PMID: 32348036
- PMCID: PMC7496261
- DOI: 10.1002/cpdd.798
Pharmacokinetics, Pharmacodynamics, Safety, and Tolerability of Dupilumab in Healthy Adult Subjects
Abstract
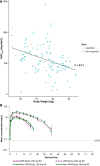
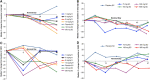
Dupilumab is a fully human monoclonal antibody directed against the interleukin (IL)-4 receptor α subunit (IL-4Rα) of IL-4 heterodimeric type I and type II receptors that mediate IL-4/IL-13 signaling through this pathway. Blockade of these receptors broadly suppresses type 2 inflammation associated with atopic/allergic diseases, including atopic dermatitis and asthma. Six phase 1 studies investigated the pharmacokinetics, pharmacodynamics, safety, and tolerability of dupilumab in healthy subjects. Two randomized, double-blind, placebo-controlled, sequential studies assessed safety and tolerability of single escalating dupilumab doses administered intravenously or subcutaneously (one included various racial groups, and one included exclusively Japanese subjects); 3 randomized, parallel-group, single-dose studies compared the pharmacokinetic profiles of different dupilumab products and formulations after single subcutaneous doses; and one study assessed dupilumab administered as fast versus slow subcutaneous injections. Dupilumab concentrations in serum were measured in all studies, and total immunoglobulin E (IgE) and thymus- and activation-regulated chemokine (TARC) concentrations were measured in 2 studies as pharmacodynamic markers. Across the phase 1 studies, dupilumab exhibited target-mediated pharmacokinetics consisting of parallel linear and nonlinear elimination, with the target-mediated phase highly dominated by nonlinearity at lower drug concentrations. Systemic exposure and tolerability of dupilumab were consistent irrespective of differences in product, formulation, or racial background. Dupilumab reduced circulating concentrations of total IgE and TARC, indicating blockade of IL-4Rα-mediated signaling. Dupilumab had a favorable safety profile across the wide range of doses administered. Together, these findings support the continued development and use of dupilumab in treatment of type 2 diseases.
Trial registration: ClinicalTrials.gov NCT01015027 NCT01537653 NCT01537640 NCT01484600.
Keywords: dupilumab; healthy subjects; pharmacodynamics; pharmacokinetics; type 2 immune diseases.
© 2020 Regeneron Pharmaceuticals, Inc. Clinical Pharmacology in Drug Development published by Wiley Periodicals LLC on behalf of American College of Clinical Pharmacology.
Conflict of interest statement
Z.L., M.L., and B.N.S. were employees of Sanofi at the time of the study and held stock and/or stock options in the company. M.K., Y.T., J.E.M., Y.L., Q.L., and C.O‐R. are employees of Sanofi and may hold stock and/or stock options in the company. A.R., J.D.H., J.D.D., A.T.D., P.K., and M.A. are employees and shareholders of Regeneron Pharmaceuticals, Inc. S.H. has nothing to disclose.
Figures


References
-
- Gandhi NA, Bennett BL, Graham NM, et al. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov. 2016;15(1):35‐50. - PubMed
-
- Peters SP, Ferguson G, Deniz Y, et al. Uncontrolled asthma: a review of the prevalence, disease burden and options for treatment. Respir Med. 2006;100(7):1139‐1151. - PubMed
-
- Bieber T. Atopic dermatitis. N Engl J Med. 2008;358(14):1483‐1494. - PubMed
-
- Passali D, Cingi C, Cambi J, et al. A survey on chronic rhinosinusitis: opinions from experts of 50 countries. Eur Arch Otorhinolaryngol. 2016;273(8):2097‐2109. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

