Inhibitory effect of a weight-loss Chinese herbal formula RCM-107 on pancreatic α-amylase activity: Enzymatic and in silico approaches
- PMID: 32348327
- PMCID: PMC7190128
- DOI: 10.1371/journal.pone.0231815
Inhibitory effect of a weight-loss Chinese herbal formula RCM-107 on pancreatic α-amylase activity: Enzymatic and in silico approaches
Abstract
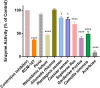
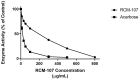
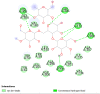
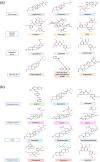
Reducing carbohydrates digestion by having a low glycaemic index (GI) foods has been linked to weight loss. Inhibiting related enzymes is an alternative way to decrease carbohydrate digestion. RCM-107 (Slimming Plus), an eight-herb formula that is modified from RCM-104, indicated significant weight-loss action in clinical trials. However, no published research has studied its mechanism of action on reducing carbohydrate absorption via suppressing the activities of porcine pancreatic alpha-amylase (PPA). In this paper, we used fluorescence PPA inhibition assay to investigate the inhibitory effects of RCM-107 and the individual herbs present in this herbal mixture on amylase activity. Subsequently, molecular docking predicted the key active compounds that may be responsible for the enzyme inhibition. According to our results, both the RCM-107 formula and several individual herbs displayed α-amylase inhibitory effects. Also, marginal synergistic effects of RCM-107 were detected. In addition, alisol B, (-)-epigallocatechin-3-gallate (EGCG) and plantagoside have been predicted as the key active compounds that may be responsible for the α-amylase inhibition effect of RCM-107 according to inter-residue contact analysis. Finally, Glu233, Gln63, His305, Asp300 and Tyr151 are predicted to be markers of important areas with which potential amylase inhibitors would interact. Therefore, our data has provided new knowledge on the mechanisms of action of the RCM-107 formula and its individual herbal ingredients for weight loss, in terms of decreasing carbohydrate digestion via the inhibition of pancreatic alpha-amylase.
Conflict of interest statement
The authors have read the journal’s policy and the authors of this manuscript have the following competing interests to declare: Tong Kang Lee Chinese Medical Centre supported this study. However, this does not alter our adherence to PLOS ONE policies on sharing data and materials. There are no patents, products in development or marketed products associated with this research to declare.
Figures


References
-
- Yan X, Zhang M, Wu T, Dai SD, Xu JL, Zhou ZK. The anti-obesity effect of green tea polysaccharides, polyphenols and caffeine in rats fed with a high-fat diet. Food Funct. 2015;6(1): 296–303. - PubMed
-
- WHO. 10 Facts on obesity: WHO; 2019 [cited 2019 18 January]. https://www.who.int/features/factfiles/obesity/en/

