Selective Modification of Tryptophan Residues in Peptides and Proteins Using a Biomimetic Electron Transfer Process
- PMID: 32348670
- PMCID: PMC7292481
- DOI: 10.1021/jacs.0c03039
Selective Modification of Tryptophan Residues in Peptides and Proteins Using a Biomimetic Electron Transfer Process
Abstract
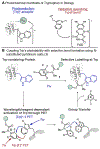
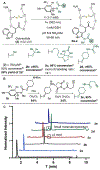
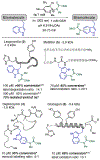
We report here a photochemical process for the selective modification of tryptophan (Trp) residues in peptides and small proteins using electron-responsive N-carbamoylpyridinium salts and UV-B light. Preliminary mechanistic experiments suggest that the photoconjugation process proceeds through photoinduced electron transfer (PET) between Trp and the pyridinium salt, followed by fragmentation of the pyridinium N-N bond and concomitant transfer of this group to Trp. The reaction displays excellent site selectivity for Trp and is tolerant to other, redox-active amino-acid residues. Moreover, the reaction proceeds in pure aqueous conditions without the requirement of organic cosolvents or photocatalysts, is enhanced by glutathione, and operates efficiently over a wide range of peptide concentrations (10-700 μM). The scope of the process was explored through the labeling of 6-Trp-containing peptides and proteins ranging from 1 to 14 kDa. We demonstrate the versatility of the N-carbamoylpyridinium salt both by tuning the electrochemical and photochemical properties of the pyridinium scaffold to enable challenging photoconjugation reactions and by using the carbamoyl moiety to tether a plethora of productive functional groups, including reactive handles, purification tags, and removable protecting groups.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
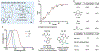

References
-
- Hermanson G Bioconjugate Techniques, 3rd ed.; Elsevier: London, 2013.
- Sletten EM; Bertozzi CR Bioorthogonal Chemistry: Fishing for Selectivity in a sea of Functionality. Angew. Chem., Int. Ed 2009, 48, 6974–6998. - PMC - PubMed
- Koniev O; Wagner A Developments and recent advancements in the field of endogenous amino acid selective bond forming reactions for bioconjugation. Chem. Soc. Rev 2015, 44, 5495–5551. - PubMed
- Boutureira O; Bernardes GJL Advances in Chemical Protein Modification. Chem. Rev 2015, 115, 2174–2195. - PubMed
- deGruyter JN; Malins LR; Baran PS Residue-Specific Modification: A Chemist’s Guide. Biochemistry 2017, 56, 3863–3873. - PMC - PubMed
-
- Takaoka Y; Ojida A; Hamachi I Protein Organic Chemistry and Applications for Labeling and Engineering in Live-Cell Systems. Angew. Chem., Int. Ed 2013, 52, 4088–4106. - PubMed
- Hu Q-Y; Berti F; Adamo R Towards the next generation of biomedicines by site-selective conjugation. Chem. Soc. Rev 2016, 45, 1691–1719. - PubMed
- Rudra A; Li J; Shakur R; Bhagchandani; Langer R Trends in Therapeutic Conjugates: Bench to Clinic. Bioconjugate Chem. 2020, 31, 462. - PubMed
- Spicer CD; Pashuck ET; Stevens MM Achieving Controlled Biomolecule-Biomaterial Conjugation. Chem. Rev 2018, 118, 7702–7743. - PMC - PubMed
-
- Bottecchia C; Noël T Photocatalytic Modification of Amino Acids, Peptides, and Proteins. Chem. - Eur. J 2019, 25, 26–42. - PMC - PubMed
- Fancy DA; Kodadek T Chemistry for the analysis of protein-protein interactions: Rapid and efficient cross-linking triggered by long wavelength light. Proc. Natl. Acad. Sci U. S. A 1999, 96, 6020–6024. - PMC - PubMed
- Kim K; Fancy DA; Carney D; Kodadek T Photoinduced Protein Cross-Linking Mediated by palladium Porphyrins. J. Am. Chem. Soc 1999, 121, 11896–11897.
- Tyson EL; Niemeyer ZL; Yoon TP Redox Mediators in Visible Light Photocatalysis: Photocalytic Radical Thiol-Ene Additions. J. Org. Chem 2014, 79, 1427–1436. - PMC - PubMed
- Vara BA; Li X; Berritt S; Walters CR; Petersson EJ; Molander GA Scalable thioarylation of unprotected peptides and biomolecules under Ni/photoredox catalysis. Chem. Sci 2018, 9, 336–344. - PMC - PubMed
- Bottecchia C; Rubens M; Gunnoo SB; Hessel V; Madder A; Noël T Visible-Light-Mediated Selective Arylation of Cysteine in Batch and Flow. Angew. Chem., Int. Ed 2017, 56, 12702–12707. - PMC - PubMed
- Sato S; Nakamura H Angew. Chem., Int. Ed 2013, 52, 8681–8684. - PubMed
- Ichiishi N; Caldwell JP; Lin M; Zhong W; Zhu X; Streckfuss E; Kim H; Parish CA; Krska SW Protecting group free radical C-H trifluoromethylation of peptides. Chem. Sci 2018, 9, 4168–4175. - PMC - PubMed
- Bloom S; Liu C; Kölmel DK; Qiao JX; Zhang Y; Poss MA; Ewing WR; MacMillan DWC Decarboxylative alkylation for site-selective bioconjugation of native proteins via oxidation potentials. Nat. Chem 2018, 10, 205–211. - PMC - PubMed
- Garreau M; Le Vaillant F; Waser J C-Terminal bioconjugation of Peptides through Photoredox Catalyzyed Decarboxylative Alkynylation. Angew. Chem., Int. Ed 2019, 58, 8182–8186. - PubMed
-
- (a) Bent DV; Hayon E Excited State Chemistry of Aromatic Amino Acids and Related Peptides. III. Tryptophan. J. Am. Chem. Soc 1975, 97, 2612–2619. - PubMed
- Creed D The Photophysics and Photochemistry of the Near-UV Absorbing Amino Acids-I. Tryptophan and its Simple Derivatives. Photochem. Photobiol 1984, 39, 577–583.
- Pattison DI; Rahmanto AS; Davies MJ Photo-oxidation of proteins. Photochem. Photobiol. Sci 2012, 11, 38–53. - PubMed
- Tsentalovich YP; Snytnikova OA; Sagdeev RZ Properties of Excited States of aquous Tryptophan. J. Photochem. Photobiol., A 2004, 162, 371–379.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

