A Strategy for Personalized Treatment of iPS-Retinal Immune Rejections Assessed in Cynomolgus Monkey Models
- PMID: 32349277
- PMCID: PMC7247695
- DOI: 10.3390/ijms21093077
A Strategy for Personalized Treatment of iPS-Retinal Immune Rejections Assessed in Cynomolgus Monkey Models
Abstract
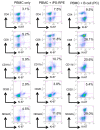
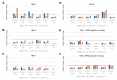
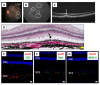
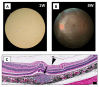
Recently, we successfully transplanted an autograft, or major histocompatibility complex (MHC)-matched allografts, from induced-pluripotent-stem-cell-derived retinal pigment epithelial (iPSC-RPE) cells in patients with age-related macular degeneration. However, there was an issue regarding immune rejection after transplantation. In this study, we established a preoperational in vitro "drug-lymphocytes-grafts immune reaction (Drug-LGIR)" test to determine the medication for immune rejection using host immunocompetent cells (lymphocytes) and transplant cells (target iPSC-RPE cells) together with different medications. The adequacy of the test was assessed by in vivo transplantation in monkey models together with medication based on in vitro data. In the results of Drug-LGIR tests, some drugs exhibited significant suppression of RPE cell-related allogeneic reactions, while other drugs did not, and the efficacy of each drug differed among the recipient monkeys. Based on the results of Drug-LGIR, we applied cyclosporine A or local steroid (triamcinolone) therapy to two monkeys, and successfully suppressed RPE-related immune rejections with RPE grafts, which survived without any signs of rejection under drug administration. We propose that our new preoperational in vitro Drug-LGIR test, which specifies the most efficacious medication for each recipient, is useful for controlling immune attacks with personalized treatment for each patient after retinal transplantation.
Keywords: drug; iPS cells; immune rejection; retinal pigment epithelial cells; transplantation.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Ohno S., Nakamura S., Hori S., Shimakawa M., Kawashima H., Mochizuki M., Sugita S., Ueno S., Yoshizaki K., Inaba G. Efficacy, safety, and pharmacokinetics of multiple administration of infliximab in Behcet’s disease with refractory uveoretinitis. J. Rheumatol. 2004;31:1362–1368. - PubMed
-
- Diaz-Llopis M., Salom D., Garcia-de-Vicuna C., Cordero-Coma M., Ortega G., Ortego N., Suarez-de-Figueroa M., Rio-Pardo M.J., Fernandez-Cid C., Fonollosa A., et al. Treatment of refractory uveitis with adalimumab: A prospective multicenter study of 131 patients. Ophthalmology. 2012;119:1575–1581. doi: 10.1016/j.ophtha.2012.02.018. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

