Telomere and Centromere Staining Followed by M-FISH Improves Diagnosis of Chromosomal Instability and Its Clinical Utility
- PMID: 32349350
- PMCID: PMC7291161
- DOI: 10.3390/genes11050475
Telomere and Centromere Staining Followed by M-FISH Improves Diagnosis of Chromosomal Instability and Its Clinical Utility
Abstract
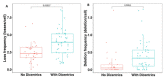
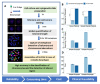
Dicentric chromosomes are a relevant marker of chromosomal instability. Their appearance is associated with telomere dysfunction, leading to cancer progression and a poor clinical outcome. Here, we present Telomere and Centromere staining followed by M-FISH (TC+M-FISH) for improved detection of telomere dysfunction and the identification of dicentric chromosomes in cancer patients and various genetic syndromes. Significant telomere length shortening and significantly higher frequencies of telomere loss and deletion were found in the peripheral lymphocytes of patients with cancer and genetic syndromes relative to similar age-matched healthy donors. We assessed our technique against conventional cytogenetics for the detection of dicentric chromosomes by subjecting metaphase preparations to both approaches. We identified dicentric chromosomes in 28/50 cancer patients and 21/44 genetic syndrome patients using our approach, but only 7/50 and 12/44, respectively, using standard cytogenetics. We ascribe this discrepancy to the identification of the unique configuration of dicentric chromosomes. We observed significantly higher frequencies of telomere loss and deletion in patients with dicentric chromosomes (p < 10-4). TC+M-FISH analysis is superior to classical cytogenetics for the detection of chromosomal instability. Our approach is a relatively simple but useful tool for documenting telomere dysfunction and chromosomal instability with the potential to become a standard additional diagnostic tool in medical genetics and the clinic.
Keywords: cancer; centromere; chromosomal instability; dicentric chromosome; genetic syndrome; telomere.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Burrell R.A., McGranahan N., Bartek J., Swanton C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature. 2013;501:338–345. - PubMed
-
- Pernot E., Hall J., Baatout S., Benotmane M.A., Blanchardon E., Bouffler S., El Saghire H., Gomolka M., Guertler A., Harms-Ringdahl M., et al. Ionizing radiation biomarkers for potential use in epidemiological studies. Mutat. Res. 2012;751:258–286. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

