Beneficial Effects of Melatonin in the Ovarian Transport Medium on In Vitro Embryo Production of Iberian Red Deer (Cervus elaphus hispanicus)
- PMID: 32349425
- PMCID: PMC7278470
- DOI: 10.3390/ani10050763
Beneficial Effects of Melatonin in the Ovarian Transport Medium on In Vitro Embryo Production of Iberian Red Deer (Cervus elaphus hispanicus)
Abstract
A major limiting factor for the development of in vitro embryo production (IVP) in wild species, such as Iberian red deer, compared to livestock animals is the poor availability and limited access to biological material. Thus, the use of post-mortem ovaries from slaughtered animals represent a source of oocytes for the large scale production of embryos needed for research and to improve the efficiency of IVP. However, these oocytes are not as developmentally competent as their in vivo counterparts. Moreover, oocytes are usually obtained from ovaries that have been transported for long distances, which may also affect their quality. In order to overcome the issues associated with prolonged storage times of post-mortem material, in this study we examined the effect of melatonin supplementation to the ovary transport medium on oocyte quality, embryo yield, and blastocyst quality in Iberian red deer. When necessary, sheep was used as an experimental model due to the large number of samples required for analysis of oocyte quality parameters. Oocytes were in vitro matured and assessed for early apoptosis; DNA fragmentation; reactive oxygen species (ROS); reduced glutathione (GSH) content, mitochondrial membrane potential, and distribution; and relative abundance of mRNA transcript levels. After in vitro fertilization, embryo rates and blastocyst quality were also investigated. The results revealed that melatonin treatment significantly increased intracellular level of GSH in sheep oocytes. Moreover, the percentage of cleavage and blastocyst yield in red deer was greater compared to the Control group and there was lower abundance of oxidative stress- and apoptosis-related SHC1, TP53, and AKR1B1 mRNA transcripts in blastocysts for the Melatonin group. In conclusion, the supplementation of melatonin to the ovary storage medium had a positive effect on the developmental competence and quality of resulting blastocysts in Iberian red deer.
Keywords: deer; embryo; melatonin; oocyte; ovary storage; sheep; transport.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
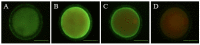




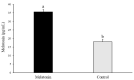


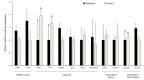
References
-
- Berg D., Thongphakdee A. Comparative Embryo Culture. Springer; Berlin, Germany: 2006. In vitro Culture of Deer Embryos; pp. 191–208. - PubMed
-
- García-Álvarez O., Maroto-Morales A., Berlinguer F., Fernández-Santos M.R., Esteso M.C., Mermillod P., Ortiz J.A., Ramon M., Pérez-Guzmán M.D., Garde J.J., et al. Effect of storage temperature during transport of ovaries on in vitro embryo production in Iberian red deer (Cervus elaphus hispanicus) Theriogenology. 2011;75:65–72. - PubMed
-
- Martinez J.G., Carranza J., Fernandez-Garcia J.L., Sanchez-Prieto C.B. Genetic Variation of Red Deer Populations under Hunting Exploitation in Southwestern Spain. J. Wildl. Manage. 2002;66:1273
-
- Garde J.J., Martínez-Pastor F., Gomendio M., Malo A.F., Soler A.J., Fernández-Santos M.R., Esteso M.C., García A.J., Anel L., Roldán E.R.S. The application of reproductive technologies to natural populations of red deer. Reprod. Domest. Anim. 2006;41:93–102. - PubMed
-
- Herrick J. Assisted reproductive technologies for endangered species conservation: developing sophisticated protocols with limited access to animals with unique reproductive mechanisms. Biol. Reprod. 2019;100:1158–1170. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

