Tissue Specific Fate of Nanomaterials by Advanced Analytical Imaging Techniques - A Review
- PMID: 32349469
- PMCID: PMC7774012
- DOI: 10.1021/acs.chemrestox.0c00072
Tissue Specific Fate of Nanomaterials by Advanced Analytical Imaging Techniques - A Review
Abstract
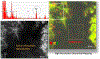
A variety of imaging and analytical methods have been developed to study nanoparticles in cells. Each has its benefits, limitations, and varying degrees of expense and difficulties in implementation. High-resolution analytical scanning transmission electron microscopy (HRSTEM) has the unique ability to image local cellular environments adjacent to a nanoparticle at near atomic resolution and apply analytical tools to these environments such as energy dispersive spectroscopy and electron energy loss spectroscopy. These tools can be used to analyze particle location, translocation and potential reformation, ion dispersion, and in vivo synthesis of second-generation nanoparticles. Such analyses can provide in depth understanding of tissue-particle interactions and effects that are caused by the environmental "invader" nanoparticles. Analytical imaging can also distinguish phases that form due to the transformation of "invader" nanoparticles in contrast to those that are triggered by a response mechanism, including the commonly observed iron biomineralization in the form of ferritin nanoparticles. The analyses can distinguish ion species, crystal phases, and valence of parent nanoparticles and reformed or in vivo synthesized phases throughout the tissue. This article will briefly review the plethora of methods that have been developed over the last 20 years with an emphasis on the state-of-the-art techniques used to image and analyze nanoparticles in cells and highlight the sample preparation necessary for biological thin section observation in a HRSTEM. Specific applications that provide visual and chemical mapping of the local cellular environments surrounding parent nanoparticles and second-generation phases are demonstrated, which will help to identify novel nanoparticle-produced adverse effects and their associated mechanisms.
Figures







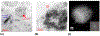




References
-
- Elder ACP, Gelein R, Finkelstein JN, Cox C, and Oberdörster G (2000) The pulmonary inflammatory response to inhaled ultrafine particles is modified by age, ozone exposure, and bacterial toxin. Inhalation Toxicol. 12, 227–246. - PubMed
-
- Oberdörster G, Finkelstein JN, Johnston C, Gelein R, Cox C, Baggs R, and Elder ACP (2000) Acute pulmonary effects of ultrafine particles in rats and mice, pp 1–74, Health Effects Institute, Cambridge, MA. - PubMed
-
- Maynard AD, Baron PA, Foley M, Shvedova AA, Kisin ER, and Castranova V (2004) Exposure to carbon nanotube material: aerosol release during the handling of unrefined single walled carbon nanotube material. J. Toxicol. Environ. Health, Part A 67 (1), 87–107. - PubMed
-
- Elder A, Gelein R, Finkelstein JN, Driscoll KE, Harkema J, and Oberdörster G (2005) Effects of subchronically inhaled carbon black in three species. I. Retention kinetics, lung inflammation, and histopathology. Toxicol. Sci 88 (2), 614–629. - PubMed
-
- Rushton EK, Jiang J, Leonard SS, Eberly S, Castranova V, Biswas P, Elder A, Han X, Gelein R, Finkelstein J, and Oberdorster G (2010) Concept of assessing nanoparticle hazards considering nanoparticle dose metric and chemical/biological response metrics. J. Toxicol. Environ. Health, Part A 73 (5), 445–61. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

