Worldwide clinical practices in perioperative antibiotic therapy for lung transplantation
- PMID: 32349719
- PMCID: PMC7191774
- DOI: 10.1186/s12890-020-1151-9
Worldwide clinical practices in perioperative antibiotic therapy for lung transplantation
Abstract
Background: Infection is the most common cause of mortality within the first year after lung transplantation (LTx). The management of perioperative antibiotic therapy is a major issue, but little is known about worldwide practices.
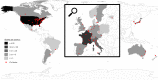
Methods: We sent by email a survey dealing with 5 daily clinical vignettes concerning perioperative antibiotic therapy to 180 LTx centers around the world. The invitation and a weekly reminder were sent to lung transplant specialists for a single consensus answer per center during a 3-month period.
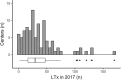
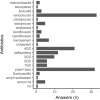
Results: We received a total of 99 responses from 24 countries, mostly from Western Europe (n = 46) and the USA (n = 34). Systematic screening for bronchial recipient colonization before LTx was mostly performed with sputum samples (72%), regardless of the underlying lung disease. In recipients without colonization, antibiotics with activity against gram-negative bacteria resistant strains (piperacillin / tazobactam, cefepime, ceftazidime, carbapenems) were reported in 72% of the centers, and antibiotics with activity against methicillin-resistant Staphylococcus aureus (mainly vancomycin) were reported in 38% of the centers. For these recipients, the duration of antibiotics reported was 7 days (33%) or less (26%) or stopped when cultures of donor and recipients were reported negatives (12%). In recipients with previous colonization, antibiotics were adapted to the susceptibility of the most resistant strain and given for at least 14 days (67%).
Conclusion: Practices vary widely around the world, but resistant bacterial strains are mostly targeted even if no colonization occurs. The antibiotic duration reported was longer for colonized recipients.
Keywords: Antibiotic therapy; Bronchial colonization; Lung transplantation; Perioperative; Survey.
Conflict of interest statement
Pr. Marc Leone discloses financial support from MSD (Merck & Co., Inc., Kenilworth, NJ, USA) and Pfizer (Paris, France). Pr. Laurent Papazian reports non-financial support from Dräger SAS France, non-financial support from Maquet SAS France, outside the submitted work. Other authors have no conflict to disclose. Pr. Federica Meloni is a member of the Editorial Board of the BMC Pulmonary Medicine journal as an Associate Editor of the section “Infectious, Rare and Idiopathic Pulmonary Diseases”.
Figures

References
-
- Yusen RD, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-second official adult lung and heart-lung transplantation report--2015; focus theme: early graft failure. J Heart Lung Transplant. 2015;34:1264–1277. - PubMed
-
- Verleden SE, Sacreas A, Vos R, Vanaudenaerde BM, Verleden GM. Advances in understanding bronchiolitis obliterans after lung transplantation. Chest. 2016;150:219–225. - PubMed
-
- Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357:2601–2614. - PubMed
-
- Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70:195–283. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

