Practical Comparison of the BioFire FilmArray Pneumonia Panel to Routine Diagnostic Methods and Potential Impact on Antimicrobial Stewardship in Adult Hospitalized Patients with Lower Respiratory Tract Infections
- PMID: 32350045
- PMCID: PMC7315039
- DOI: 10.1128/JCM.00135-20
Practical Comparison of the BioFire FilmArray Pneumonia Panel to Routine Diagnostic Methods and Potential Impact on Antimicrobial Stewardship in Adult Hospitalized Patients with Lower Respiratory Tract Infections
Abstract
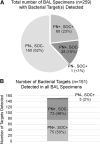
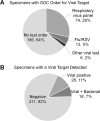
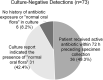
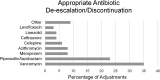
Lower respiratory tract infections, including hospital-acquired and ventilator-associated pneumonia, are common in hospitalized patient populations. Standard methods frequently fail to identify the infectious etiology due to the polymicrobial nature of respiratory specimens and the necessity of ordering specific tests to identify viral agents. The potential severity of these infections combined with a failure to clearly identify the causative pathogen results in administration of empirical antibiotic agents based on clinical presentation and other risk factors. We examined the impact of the multiplexed, semiquantitative BioFire FilmArray Pneumonia panel (PN panel) test on laboratory reporting for 259 adult inpatients submitting bronchoalveolar lavage (BAL) specimens for laboratory analysis. The PN panel demonstrated a combined 96.2% positive percent agreement (PPA) and 98.1% negative percent agreement (NPA) for the qualitative identification of 15 bacterial targets compared to routine bacterial culture. Semiquantitative values reported by the PN panel were frequently higher than values reported by culture, resulting in semiquantitative agreement (within the same log10 value) of 43.6% between the PN panel and culture; however, all bacterial targets reported as >105 CFU/ml in culture were reported as ≥105 genomic copies/ml by the PN panel. Viral targets were identified by the PN panel in 17.7% of specimens tested, of which 39.1% were detected in conjunction with a bacterial target. A review of patient medical records, including clinically prescribed antibiotics, revealed the potential for antibiotic adjustment in 70.7% of patients based on the PN panel result, including discontinuation or de-escalation in 48.2% of patients, resulting in an average savings of 6.2 antibiotic days/patient.
Keywords: medical outcomes; multiplex; pneumonia; stewardship.
Copyright © 2020 Buchan et al.
Figures
References
-
- GBD 2013 Mortality and Causes of Death Collaborators. 2015. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 385:117–171. doi:10.1016/S0140-6736(14)61682-2. - DOI - PMC - PubMed
-
- Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O'Grady NP, Bartlett JG, Carratalà J, El Solh AA, Ewig S, Fey PD, File TM, Restrepo MI, Roberts JA, Waterer GW, Cruse P, Knight SL, Brozek JL. 2016. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 63:e61–e111. doi:10.1093/cid/ciw353. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

