Metabolic regulation of the lysosomal cofactor bis(monoacylglycero)phosphate in mice
- PMID: 32350080
- PMCID: PMC7328040
- DOI: 10.1194/jlr.RA119000516
Metabolic regulation of the lysosomal cofactor bis(monoacylglycero)phosphate in mice
Abstract
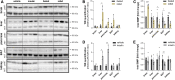
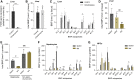
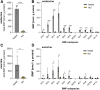
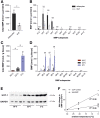
Bis(monoacylglycero)phosphate (BMP), also known as lysobisphosphatidic acid, is a phospholipid that promotes lipid sorting in late endosomes/lysosomes by activating lipid hydrolases and lipid transfer proteins. Changes in the cellular BMP content therefore reflect an altered metabolic activity of the endolysosomal system. Surprisingly, little is known about the physiological regulation of BMP. In this study, we investigated the effects of nutritional and metabolic factors on BMP profiles of whole tissues and parenchymal and nonparenchymal cells. Tissue samples were obtained from fed, fasted, 2 h refed, and insulin-treated mice, as well as from mice housed at 5°C, 22°C, or 30°C. These tissues exhibited distinct BMP profiles that were regulated by the nutritional state in a tissue-specific manner. Insulin treatment was not sufficient to mimic refeeding-induced changes in tissue BMP levels, indicating that BMP metabolism is regulated by other hormonal or nutritional factors. Tissue fractionation experiments revealed that fasting drastically elevates BMP levels in hepatocytes and pancreatic cells. Furthermore, we observed that the BMP content in brown adipose tissue strongly depends on housing temperatures. In conclusion, our observations suggest that BMP concentrations adapt to the metabolic state in a tissue- and cell-type-specific manner in mice. Drastic changes observed in hepatocytes, pancreatic cells, and brown adipocytes suggest that BMP plays a role in the functional adaption to nutrient starvation and ambient temperature.
Keywords: adipose tissue; body temperature; insulin; lipid metabolism; liver; lysosome; nutritional state; pancreas; phospholipids.
Copyright © 2020 Grabner et al. Published by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures

References
-
- Kobayashi T., Beuchat M-H., Chevallier J., Makino A., Mayran N., Escola J-M., Lebrand C., Cosson P., Kobayashi T., and Gruenberg J.. 2002. Separation and characterization of late endosomal membrane domains. J. Biol. Chem. 277: 32157–32164. - PubMed
-
- Tan H. H., Makino A., Sudesh K., Greimel P., and Kobayashi T.. 2012. Spectroscopic evidence for the unusual stereochemical configuration of an endosome-specific lipid. Angew. Chem. Int. Ed. Engl. 51: 533–535. - PubMed
-
- Schulze H., Kolter T., and Sandhoff K.. 2009. Principles of lysosomal membrane degradation: cellular topology and biochemistry of lysosomal lipid degradation. Biochim. Biophys. Acta 1793: 674–683. - PubMed
-
- Locatelli-Hoops S., Remmel N., Klingenstein R., Breiden B., Rossocha M., Schoeniger M., Koenigs C., Saenger W., and Sandhoff K.. 2006. Saposin A mobilizes lipids from low cholesterol and high bis(monoacylglycerol)phosphate-containing membranes: patient variant Saposin A lacks lipid extraction capacity. J. Biol. Chem. 281: 32451–32460. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

