Incomplete Freund's adjuvant reduces arginase and enhances Th1 dominance, TLR signaling and CD40 ligand expression in the vaccine site microenvironment
- PMID: 32350119
- PMCID: PMC7213888
- DOI: 10.1136/jitc-2020-000544
Incomplete Freund's adjuvant reduces arginase and enhances Th1 dominance, TLR signaling and CD40 ligand expression in the vaccine site microenvironment
Abstract
Background: Immunogenicity of cancer vaccines is impacted by adjuvants and schedule, but systematic assessments of their effects have not been performed. Montanide ISA-51, an incomplete Freund's adjuvant (IFA), is used in many vaccine trials, but concerns have been raised about negative effects in murine studies. We found in humans that IFA enhances systemic immune responses and that repeat vaccination at one site (same site vaccination (SSV)) creates tertiary lymphoid structures (TLS) in the vaccine site microenvironment (VSME). We hypothesized that vaccination with peptides+IFA+pICLC or SSV×3 with peptides in IFA would create an immunogenic milieu locally at the VSME, with activated dendritic cells (DC), TLS-associated chemokines and a Th1-dominant VSME.
Methods: Biopsies of the VSME were obtained from participants on two clinical trials who were immunized with multiple melanoma peptides (MELITAC 12.1) in adjuvants comprising IFA and/or the TLR3-agonist pICLC. Biopsies were obtained either a week after one vaccine or a week after SSV×3. Controls included normal skin and skin injected with IFA without peptides. Gene expression analysis was performed by RNAseq.
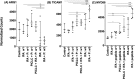
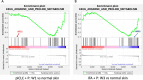
Results: VSME samples were evaluated from 27 patients. One vaccine with peptides in pICLC+IFA enhanced expression of CD80, CD83, CD86 (p<0.01), CD40 and CD40L (p<0.0001) over normal skin; these effects were significantly enhanced for SSV with peptides+IFA. Vaccines containing pICLC increased expression of TBX21 (T-bet) but did not decrease GATA3 over normal skin, whereas SSV with peptides in IFA dramatically enhanced TBX21 and decreased GATA3, with high expression of IFNγ and STAT1. SSV with peptides in IFA also reduced arginase-1 (ARG1) expression and enhanced expression of TLR adapter molecules TICAM-1 (TRIF) and MYD88. Furthermore, SSV with IFA and peptides also enhanced expression of chemokines associated with TLS formation.
Conclusions: These findings suggest that SSV with peptides in IFA enhances CD40L expression by CD4 T cells, supports a Th1 microenvironment, with accumulation of activated and mature DC. Increased expression of TLR adaptor proteins after SSV with peptides in IFA might implicate effects of the skin microbiome. Reduced ARG1 may reflect diminished suppressive myeloid activity in the VSME.
Trial registration number: (NCT00705640, NCT01585350).
Keywords: adjuvants, pharmaceutic; arginase; cytokines; immunogenicity, vaccine; melanoma.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: CLSJ is listed as a co-inventor for some of the peptides used in the 12MP vaccine. The patents are held by the UVA Licensing and Ventures group (LVG).
Figures




References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
