Effective treatment of severe COVID-19 patients with tocilizumab
- PMID: 32350134
- PMCID: PMC7245089
- DOI: 10.1073/pnas.2005615117
Effective treatment of severe COVID-19 patients with tocilizumab
Abstract
After analyzing the immune characteristics of patients with severe coronavirus disease 2019 (COVID-19), we have identified that pathogenic T cells and inflammatory monocytes with large amount of interleukin 6 secreting may incite the inflammatory storm, which may potentially be curbed through monoclonal antibody that targets the IL-6 pathways. Here, we aimed to assess the efficacy of tocilizumab in severe patients with COVID-19 and seek a therapeutic strategy. The patients diagnosed as severe or critical COVID-19 in The First Affiliated Hospital of University of Science and Technology of China (Anhui Provincial Hospital) and Anhui Fuyang Second People's Hospital were given tocilizumab in addition to routine therapy between 5 and 14 February 2020. The changes of clinical manifestations, computerized tomography (CT) scan image, and laboratory examinations were retrospectively analyzed. Fever returned to normal on the first day, and other symptoms improved remarkably within a few days. Within 5 d after tocilizumab, 15 of the 20 patients (75.0%) had lowered their oxygen intake, and 1 patient needed no oxygen therapy. CT scans manifested that the lung lesion opacity absorbed in 19 patients (90.5%). The percentage of lymphocytes in peripheral blood, which decreased in 85.0% of patients (17/20) before treatment (mean, 15.52 ± 8.89%), returned to normal in 52.6% of patients (10/19) on the fifth day after treatment. Abnormally elevated C-reactive protein decreased significantly in 84.2% of patients (16/19). No obvious adverse reactions were observed. All patients have been discharged on average 15.1 d after giving tocilizumab. Preliminary data show that tocilizumab, which improved the clinical outcome immediately in severe and critical COVID-19 patients, is an effective treatment to reduce mortality.
Keywords: COVID-19; SARS-CoV-2; cytokine storm; interleukin-6; tocilizumab.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures
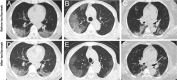
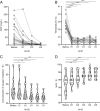
Comment in
-
Favorable changes of CT findings in a patient with COVID-19 pneumonia after treatment with tocilizumab.Diagn Interv Imaging. 2020 May;101(5):323-324. doi: 10.1016/j.diii.2020.03.010. Epub 2020 Mar 31. Diagn Interv Imaging. 2020. PMID: 32278585 Free PMC article. No abstract available.
-
Are there any association between COVID-19 severity and immunosuppressive therapy?Immunol Lett. 2020 Aug;224:12-13. doi: 10.1016/j.imlet.2020.05.002. Epub 2020 May 28. Immunol Lett. 2020. PMID: 32473971 Free PMC article. No abstract available.
-
Safety concerns regarding concomitant use of tocilizumab and glucocorticoids in COVID-19 patients.Proc Natl Acad Sci U S A. 2020 Dec 1;117(48):30025-30026. doi: 10.1073/pnas.2009253117. Epub 2020 Nov 12. Proc Natl Acad Sci U S A. 2020. PMID: 33184179 Free PMC article. No abstract available.
-
Reply to Wang et al.: Tocilizumab treatment should be used in a timely manner, at suitable dose, and in suitable patients.Proc Natl Acad Sci U S A. 2020 Dec 8;117(49):30898-30899. doi: 10.1073/pnas.2017204117. Epub 2020 Nov 17. Proc Natl Acad Sci U S A. 2020. PMID: 33203670 Free PMC article. No abstract available.
-
Does tocilizumab have a magical therapeutic effect on COVID-19 patients without obvious adverse reactions?Proc Natl Acad Sci U S A. 2020 Dec 8;117(49):30896-30897. doi: 10.1073/pnas.2009961117. Epub 2020 Nov 17. Proc Natl Acad Sci U S A. 2020. PMID: 33203671 Free PMC article. No abstract available.
References
-
- Gorbalenya A. E., et al. , Severe acute respiratory syndrome-related coronavirus: The species and its viruses—a statement of the Coronavirus Study Group. 10.1101/2020.02.07.937862 (11 February 2020). - DOI
-
- World Health Organization , WHO Director-General’s remarks at the media briefing on 2019-nCoV (2020). https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at.... Accessed 11 February 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

