Myosin-VIIa is expressed in multiple isoforms and essential for tensioning the hair cell mechanotransduction complex
- PMID: 32350269
- PMCID: PMC7190839
- DOI: 10.1038/s41467-020-15936-z
Myosin-VIIa is expressed in multiple isoforms and essential for tensioning the hair cell mechanotransduction complex
Abstract
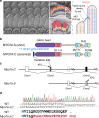
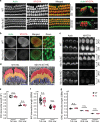
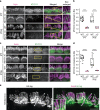
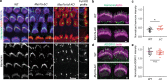
Mutations in myosin-VIIa (MYO7A) cause Usher syndrome type 1, characterized by combined deafness and blindness. MYO7A is proposed to function as a motor that tensions the hair cell mechanotransduction (MET) complex, but conclusive evidence is lacking. Here we report that multiple MYO7A isoforms are expressed in the mouse cochlea. In mice with a specific deletion of the canonical isoform (Myo7a-ΔC mouse), MYO7A is severely diminished in inner hair cells (IHCs), while expression in outer hair cells is affected tonotopically. IHCs of Myo7a-ΔC mice undergo normal development, but exhibit reduced resting open probability and slowed onset of MET currents, consistent with MYO7A's proposed role in tensioning the tip link. Mature IHCs of Myo7a-ΔC mice degenerate over time, giving rise to progressive hearing loss. Taken together, our study reveals an unexpected isoform diversity of MYO7A expression in the cochlea and highlights MYO7A's essential role in tensioning the hair cell MET complex.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

