Upregulation of CD38 expression on multiple myeloma cells by novel HDAC6 inhibitors is a class effect and augments the efficacy of daratumumab
- PMID: 32350373
- PMCID: PMC8318885
- DOI: 10.1038/s41375-020-0840-y
Upregulation of CD38 expression on multiple myeloma cells by novel HDAC6 inhibitors is a class effect and augments the efficacy of daratumumab
Erratum in
-
Publisher Correction: Upregulation of CD38 expression on multiple myeloma cells by novel HDAC6 inhibitors is a class effect and augments the efficacy of daratumumab.Leukemia. 2022 Jan;36(1):297. doi: 10.1038/s41375-021-01355-6. Leukemia. 2022. PMID: 34845317 Free PMC article. No abstract available.
Abstract
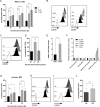
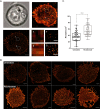
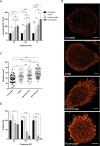
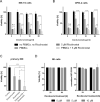
Multiple myeloma (MM) is incurable, so there is a significant unmet need for effective therapy for patients with relapsed or refractory disease. This situation has not changed despite the recent approval of the anti-CD38 antibody daratumumab, one of the most potent agents in MM treatment. The efficiency of daratumumab might be improved by combining it with synergistic anti-MM agents. We therefore investigated the potential of the histone deacetylase (HDAC) inhibitor ricolinostat to up-regulate CD38 on MM cells, thereby enhancing the performance of CD38-specific therapies. Using quantitative reverse transcription polymerase chain reaction and flow cytometry, we observed that ricolinostat significantly increases CD38 RNA levels and CD38 surface expression on MM cells. Super-resolution microscopy imaging of MM cells by direct stochastic optical reconstruction microscopy confirmed this rise with molecular resolution and revealed homogeneous distribution of CD38 molecules on the cell membrane. Particularly important is that combining ricolinostat with daratumumab induced enhanced lysis of MM cells. We also evaluated next-generation HDAC6 inhibitors (ACY-241, WT-161) and observed similar increase of CD38 levels suggesting that the upregulation of CD38 expression on MM cells by HDAC6 inhibitors is a class effect. This proof-of-concept illustrates the potential benefit of combining HDAC6 inhibitors and CD38-directed immunotherapy for MM treatment.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Dimopoulos MA, Beksac M, Benboubker L, Roddie H, Allietta N, Broer E, et al. Phase II study of bortezomib-dexamethasone alone or with added cyclophosphamide or lenalidomide for sub-optimal response as second-line treatment for patients with multiple myeloma. Haematologica. 2013;98:1264–72. - PMC - PubMed
-
- Ayed AO, Chang LJ, Moreb JS. Immunotherapy for multiple myeloma: current status and future directions. Crit Rev Oncol/Hematol. 2015;96:399–412. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

