Rethinking the role of hydroxychloroquine in the treatment of COVID-19
- PMID: 32350928
- PMCID: PMC7267640
- DOI: 10.1096/fj.202000919
Rethinking the role of hydroxychloroquine in the treatment of COVID-19
Abstract
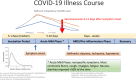
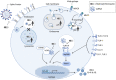
There are currently no proven or approved treatments for coronavirus disease 2019 (COVID-19). Early anecdotal reports and limited in vitro data led to the significant uptake of hydroxychloroquine (HCQ), and to lesser extent chloroquine (CQ), for many patients with this disease. As an increasing number of patients with COVID-19 are treated with these agents and more evidence accumulates, there continues to be no high-quality clinical data showing a clear benefit of these agents for this disease. Moreover, these agents have the potential to cause harm, including a broad range of adverse events including serious cardiac side effects when combined with other agents. In addition, the known and potent immunomodulatory effects of these agents which support their use in the treatment of auto-immune conditions, and provided a component in the original rationale for their use in patients with COVID-19, may, in fact, undermine their utility in the context of the treatment of this respiratory viral infection. Specifically, the impact of HCQ on cytokine production and suppression of antigen presentation may have immunologic consequences that hamper innate and adaptive antiviral immune responses for patients with COVID-19. Similarly, the reported in vitro inhibition of viral proliferation is largely derived from the blockade of viral fusion that initiates infection rather than the direct inhibition of viral replication as seen with nucleoside/tide analogs in other viral infections. Given these facts and the growing uncertainty about these agents for the treatment of COVID-19, it is clear that at the very least thoughtful planning and data collection from randomized clinical trials are needed to understand what if any role these agents may have in this disease. In this article, we review the datasets that support or detract from the use of these agents for the treatment of COVID-19 and render a data informed opinion that they should only be used with caution and in the context of carefully thought out clinical trials, or on a case-by-case basis after rigorous consideration of the risks and benefits of this therapeutic approach.
Keywords: COV-SARS-2; SARS; coronavirus; immune; immunology.
© 2020 The Authors. The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

