Actinobacterial Degradation of 2-Hydroxyisobutyric Acid Proceeds via Acetone and Formyl-CoA by Employing a Thiamine-Dependent Lyase Reaction
- PMID: 32351493
- PMCID: PMC7176365
- DOI: 10.3389/fmicb.2020.00691
Actinobacterial Degradation of 2-Hydroxyisobutyric Acid Proceeds via Acetone and Formyl-CoA by Employing a Thiamine-Dependent Lyase Reaction
Abstract
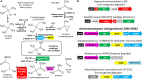
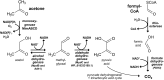
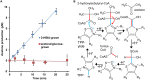
The tertiary branched short-chain 2-hydroxyisobutyric acid (2-HIBA) has been associated with several metabolic diseases and lysine 2-hydroxyisobutyrylation seems to be a common eukaryotic as well as prokaryotic post-translational modification in proteins. In contrast, the underlying 2-HIBA metabolism has thus far only been detected in a few microorganisms, such as the betaproteobacterium Aquincola tertiaricarbonis L108 and the Bacillus group bacterium Kyrpidia tusciae DSM 2912. In these strains, 2-HIBA can be specifically activated to the corresponding CoA thioester by the 2-HIBA-CoA ligase (HCL) and is then isomerized to 3-hydroxybutyryl-CoA in a reversible and B12-dependent mutase reaction. Here, we demonstrate that the actinobacterial strain Actinomycetospora chiangmaiensis DSM 45062 degrades 2-HIBA and also its precursor 2-methylpropane-1,2-diol via acetone and formic acid by employing a thiamine pyrophosphate-dependent lyase. The corresponding gene is located directly upstream of hcl, which has previously been found only in operonic association with the 2-hydroxyisobutyryl-CoA mutase genes in other bacteria. Heterologous expression of the lyase gene from DSM 45062 in E. coli established a 2-hydroxyisobutyryl-CoA lyase activity in the latter. In line with this, analysis of the DSM 45062 proteome reveals a strong induction of the lyase-HCL gene cluster on 2-HIBA. Acetone is likely degraded via hydroxylation to acetol catalyzed by a MimABCD-related binuclear iron monooxygenase and formic acid appears to be oxidized to CO2 by selenium-dependent dehydrogenases. The presence of the lyase-HCL gene cluster in isoprene-degrading Rhodococcus strains and Pseudonocardia associated with tropical leafcutter ant species points to a role in degradation of biogenic short-chain ketones and highly branched organic compounds.
Keywords: 2-hydroxyacyl-CoA lyase; Mycolicibacterium; acyloin condensation; degradation pathway; fuel oxygenate; isobutene; tert-butyl alcohol.
Copyright © 2020 Rohwerder, Rohde, Jehmlich and Purswani.
Figures


References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

