The Immune Response to the fVIII Gene Therapy in Preclinical Models
- PMID: 32351497
- PMCID: PMC7174743
- DOI: 10.3389/fimmu.2020.00494
The Immune Response to the fVIII Gene Therapy in Preclinical Models
Abstract
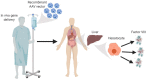
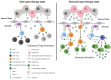
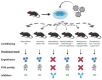
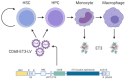
Neutralizing antibodies to factor VIII (fVIII), referred to as "inhibitors," remain the most challenging complication post-fVIII replacement therapy. Preclinical development of novel fVIII products involves studies incorporating hemophilia A (HA) and wild-type animal models. Though immunogenicity is a critical aspect of preclinical pharmacology studies, gene therapy studies tend to focus on fVIII expression levels without major consideration for immunogenicity. Therefore, little clarity exists on whether preclinical testing can be predictive of clinical immunogenicity risk. Despite this, but perhaps due to the potential for transformative benefits, clinical gene therapy trials have progressed rapidly. In more than two decades, no inhibitors have been observed. However, all trials are conducted in previously treated patients without a history of inhibitors. The current review thus focuses on our understanding of preclinical immunogenicity for HA gene therapy candidates and the potential indication for inhibitor treatment, with a focus on product- and platform-specific determinants, including fVIII transgene sequence composition and tissue/vector biodistribution. Currently, the two leading clinical gene therapy vectors are adeno-associated viral (AAV) and lentiviral (LV) vectors. For HA applications, AAV vectors are liver-tropic and employ synthetic, high-expressing, liver-specific promoters. Factors including vector serotype and biodistribution, transcriptional regulatory elements, transgene sequence, dosing, liver immunoprivilege, and host immune status may contribute to tipping the scale between immunogenicity and tolerance. Many of these factors can also be important in delivery of LV-fVIII gene therapy, especially when delivered intravenously for liver-directed fVIII expression. However, ex vivo LV-fVIII targeting and transplantation of hematopoietic stem and progenitor cells (HSPC) has been demonstrated to achieve durable and curative fVIII production without inhibitor development in preclinical models. A critical variable appears to be pre-transplantation conditioning regimens that suppress and/or ablate T cells. Additionally, we and others have demonstrated the potential of LV-fVIII HSPC and liver-directed AAV-fVIII gene therapy to eradicate pre-existing inhibitors in murine and canine models of HA, respectively. Future preclinical studies will be essential to elucidate immune mechanism(s) at play in the context of gene therapy for HA, as well as strategies for preventing adverse immune responses and promoting immune tolerance even in the setting of pre-existing inhibitors.
Keywords: adeno-associated viral vectors; factor VIII (fVIII); gene therapy; hematopoietic (stem) cells; hemophilia A; inhibitors; lentiviral (LV) vector.
Copyright © 2020 Patel, Lundgren, Spencer and Doering.
Figures
References
-
- Lollar P. Pathogenic antibodies to coagulation factors. Part one: factor VIII and factor IX. J Thromb Haemost. (2004) 2:1082–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

