Enantioselective Electroreductive Coupling of Alkenyl and Benzyl Halides via Nickel Catalysis
- PMID: 32351776
- PMCID: PMC7190267
- DOI: 10.1021/acscatal.9b01785
Enantioselective Electroreductive Coupling of Alkenyl and Benzyl Halides via Nickel Catalysis
Abstract
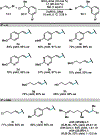
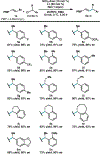
An electrochemically-driven enantioselective nickel-catalyzed reductive cross-coupling of alkenyl bromides and benzyl chlorides is reported. The reaction forms products bearing allylic stereogenic centers with good enantioselectivity under mild conditions in an undivided cell. Electrochemical activation and turnover of the catalyst mitigate issues posed by metal powder reductants. This report demonstrates that enantioselective Ni-catalyzed cross-electrophile couplings can be driven electrochemically.
Keywords: Nickel; asymmetric catalysis; cross-coupling; electrochemistry; enantioselective.
Figures

References
-
- Knappke CEI; Grupe S; Gärtner D; Corpet M; Gosmini C; Jacobi von Wangelin A Reductive Cross-Coupling Reactions between Two Electrophiles. Chem. Eur. J 2014, 20, 6828–6842. - PubMed
- Everson DA; Weix DJ Cross-Electrophile Coupling: Principles of Reactivity and Selectivity. J. Org. Chem 2014, 79, 4793–4798. - PMC - PubMed
- Weix DJ Methods and Mechanisms for Cross-Electrophile Coupling of Csp2 Halides with Alkyl Electrophiles. Acc. Chem. Res 2015, 48, 1767–1775. - PMC - PubMed
- Wang X; Dai Y; Gong H Nickel-Catalyzed Reductive Couplings. Top. Curr. Chem. (Z) 2016, 374, 43. - PubMed
-
- Cherney AH; Kadunce NT; Reisman SE Catalytic Asymmetric Reductive Acyl Cross-Coupling: Synthesis of Enantioenriched Acyclic α,α-Disubstituted Ketones. J. Am. Chem. Soc. 2013, 135, 7442–7445. - PubMed
- Cherney AH; Reisman SE Nickel-Catalyzed Asymmetric Reductive Cross-Coupling Between Vinyl and Benzyl Electrophiles. J. Am. Chem. Soc 2014, 136, 14365–14368. - PMC - PubMed
- Kadunce NT; Reisman SE Nickel-Catalyzed Asymmetric Reductive Cross-Coupling between Heteroaryl Iodides and α-Chloronitriles. J. Am. Chem. Soc 2015, 137, 10480–10483. - PMC - PubMed
- Poremba KE; Kadunce NT; Suzuki N; Cherney AH; Reisman SE Nickel-Catalyzed Asymmetric Reductive Cross-Coupling to Access 1,1-Diarylalkanes. J. Am. Chem. Soc 2017, 139, 5684–5687. - PMC - PubMed
- Hofstra JL; Cherney AH; Ordner CM; Reisman SE Synthesis of Enantioenriched Allylic Silanes via Nickel-Catalyzed Reductive Cross-Coupling. J. Am. Chem. Soc 2018, 140, 139–142. - PMC - PubMed
-
- For a review, see: Lucas EL; Jarvo ER Stereospecific and Stereoconvergent Cross-Couplings between Alkyl Electrophiles. Nature Rev. Chem 2017, 1, 0065.
-
- Anka-Lufford LL; Huihui KMM; Gower NJ; Ackerman LKG; Weix DJ Nickel-Catalyzed Cross-Electrophile Coupling with Organic Reductants in Non-Amide Solvents. Chem. Eur. J 2016, 22, 11564–11567. - PubMed
- García-Domínguez A; Li Z; Nevado C Nickel-Catalyzed Reductive Dicarbofunctionalization of Alkenes. J. Am. Chem. Soc 2017, 139, 6835–6838. - PubMed
- (c)Xu H; Zhao C; Qian Q; Deng W; Gong H Nickel-Catalyzed Cross-Coupling of Unactivated Alkyl Halides Using Bis(Pinacolato)Diboron as Reductant. Chem. Sci 2013, 4, 4022–4029.
-
- Zhang P; Le C. “Chip”; MacMillan DWC. Silyl Radical Activation of Alkyl Halides in Metallaphotoredox Catalysis: A Unique Pathway for Cross-Electrophile Coupling. J. Am. Chem. Soc 2016, 138, 8084–8087. - PMC - PubMed
- Smith RT; Zhang Xiaheng, Rincón JA; Agejas J; Materos C; Barberis M; García-Cerrada, de Frutos O; MacMillan DWC Metallaphotoredox-Catalyzed Cross-Electrophile Csp3–Csp3 Coupling. J. Am. Chem. Soc 2018, 140, 17433–17438. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
