Sinapic Acid Promotes Browning of 3T3-L1 Adipocytes via p38 MAPK/CREB Pathway
- PMID: 32351999
- PMCID: PMC7171644
- DOI: 10.1155/2020/5753623
Sinapic Acid Promotes Browning of 3T3-L1 Adipocytes via p38 MAPK/CREB Pathway
Abstract
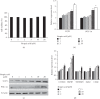
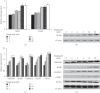
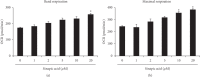
Sinapic acid is a plant-derived phenolic compound, which acts as an antioxidant, anticancer, and anti-inflammatory agent. Although sinapic acid is valuable in a variety of therapeutic applications, its role in the improvement of obesity-related metabolic disease is relatively unexplored. Brown-like adipocytes (beige adipocytes) are characterized by a high concentration of mitochondria and high expression of uncoupling protein 1 (UCP1), which has specific functions in energy expenditure and thermogenesis. This study assessed the browning effects of sinapic acid in 3T3-L1 adipocytes. We investigated the expression of beige marker genes in 3T3-L1 adipocytes treated with sinapic acid. Sinapic acid increased the expression of peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) and UCP1. Sinapic acid also promoted mitochondrial biogenesis by dose-dependently upregulating the oxygen consumption rate and enhancing the expression of representative subunits of oxidative phosphorylation complexes. In addition, treatment with p38 mitogen-activated protein kinase (MAPK) inhibitor and cAMP response element binding (CREB) inhibitor decreased the expressions of genes associated with thermogenesis, mitochondrial biogenesis, and oxidative phosphorylation. In summary, sinapic acid initiates browning 3T3-L1 adipocytes via the p38 MAPK/CREB signaling pathway. Thus, sinapic acid may have potential therapeutic implication in obesity.
Copyright © 2020 In-Seon Bae and Sang Hoon Kim.
Conflict of interest statement
The authors report no conflicts of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

