Tunneling Nanotubes and the Eye: Intercellular Communication and Implications for Ocular Health and Disease
- PMID: 32352005
- PMCID: PMC7171654
- DOI: 10.1155/2020/7246785
Tunneling Nanotubes and the Eye: Intercellular Communication and Implications for Ocular Health and Disease
Abstract
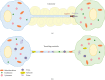
Cellular communication is an essential process for the development and maintenance of all tissues including the eye. Recently, a new method of cellular communication has been described, which relies on formation of tubules, called tunneling nanotubes (TNTs). These structures connect the cytoplasm of adjacent cells and allow the direct transport of cellular cargo between cells without the need for secretion into the extracellular milieu. TNTs may be an important mechanism for signaling between cells that reside long distances from each other or for cells in aqueous environments, where diffusion-based signaling is challenging. Given the wide range of cargoes transported, such as lysosomes, endosomes, mitochondria, viruses, and miRNAs, TNTs may play a role in normal homeostatic processes in the eye as well as function in ocular disease. This review will describe TNT cellular communication in ocular cell cultures and the mammalian eye in vivo, the role of TNTs in mitochondrial transport with an emphasis on mitochondrial eye diseases, and molecules involved in TNT biogenesis and their function in eyes, and finally, we will describe TNT formation in inflammation, cancer, and stem cells, focusing on pathological processes of particular interest to vision scientists.
Copyright © 2020 Holly R. Chinnery and Kate E. Keller.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

