Mesenchymal stromal cells induce regulatory T cells via epigenetic conversion of human conventional CD4 T cells in vitro
- PMID: 32352186
- PMCID: PMC7497276
- DOI: 10.1002/stem.3185
Mesenchymal stromal cells induce regulatory T cells via epigenetic conversion of human conventional CD4 T cells in vitro
Abstract
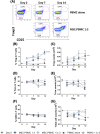
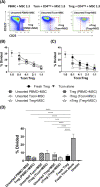
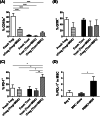
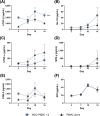
Regulatory T cells (Treg) play a critical role in immune tolerance. The scarcity of Treg therapy clinical trials in humans has been largely due to the difficulty in obtaining sufficient Treg numbers. We performed a preclinical investigation on the potential of mesenchymal stromal cells (MSCs) to expand Treg in vitro to support future clinical trials. Human peripheral blood mononuclear cells from healthy donors were cocultured with allogeneic bone marrow-derived MSCs expanded under xenogeneic-free conditions. Our data show an increase in the counts and frequency of CD4+ CD25high Foxp3+ CD127low Treg cells (4- and 6-fold, respectively) after a 14-day coculture. However, natural Treg do not proliferate in coculture with MSCs. When purified conventional CD4 T cells (Tcon) are cocultured with MSCs, only cells that acquire a Treg-like phenotype proliferate. These MSC-induced Treg-like cells also resemble Treg functionally, since they suppress autologous Tcon proliferation. Importantly, the DNA methylation profile of MSC-induced Treg-like cells more closely resembles that of natural Treg than of Tcon, indicating that this population is stable. The expression of PD-1 is higher in Treg-like cells than in Tcon, whereas the frequency of PDL-1 increases in MSCs after coculture. TGF-β levels are also significantly increased MSC cocultures. Overall, our data suggest that Treg enrichment by MSCs results from Tcon conversion into Treg-like cells, rather than to expansion of natural Treg, possibly through mechanisms involving TGF-β and/or PD-1/PDL-1 expression. This MSC-induced Treg population closely resembles natural Treg in terms of phenotype, suppressive ability, and methylation profile.
Keywords: DNA methylation; cell- and tissue-based therapy; mesenchymal stromal cells; regulatory T lymphocytes.
©2020 The Authors. Stem Cells published by Wiley Periodicals LLC on behalf of AlphaMed Press.
Conflict of interest statement
The authors declared no potential conflicts of interest.
Figures



References
-
- Di Ianni M, Falzetti F, Carotti A, et al. Tregs prevent GVHD and promote immune reconstitution in HLA‐haploidentical transplantation. Blood. 2011;117:3921‐3928. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

