Plasmodium berghei Gamete Egress Protein is required for fertility of both genders
- PMID: 32352241
- PMCID: PMC7349110
- DOI: 10.1002/mbo3.1038
Plasmodium berghei Gamete Egress Protein is required for fertility of both genders
Abstract
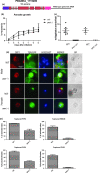
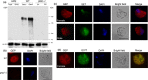
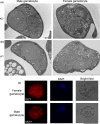
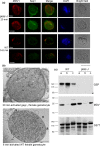
Male and female Plasmodium gametocytes ingested by the Anopheles mosquitoes during a blood meal egress from the red blood cells by rupturing the two surrounding membranes, the parasitophorous vacuole and the red blood cell membranes. Proteins of the so-called osmiophilic bodies, (OBs), secretory organelles resident in the cytoplasm, are important players in this process. Once gametes emerge, the female is ready to be fertilized while the male develops into motile flagellar gametes. Here, we describe the function(s) of PBANKA_1115200, which we named Gamete Egress Protein (GEP), a protein specific to malaria parasites. GEP is restricted to gametocytes, expressed in gametocytes of both genders and partly localizes to the OBs. A mutant lacking the protein shows aberrant rupture of the two surrounding membranes, while OBs discharge is delayed but not aborted. Moreover, we identified a second function of GEP during exflagellation since the axonemes of the male flagellar gametes were not motile. Genetic crossing experiments reveal that both genders are unable to establish infections in mosquitoes and thus the lack of GEP leads to a complete block in Plasmodium transmission from mice to mosquitoes. The combination of our results reveals essential and pleiotropic functions of GEP in Plasmodium gametogenesis.
Keywords: Anopheles; gametocyte egress; malaria; osmiophilic bodies.
© 2020 The Authors. MicrobiologyOpen published by John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures


References
-
- Andreadaki, M. , Hanssen, E. , Deligianni, E. , Claudet, C. , Wengelnik, K. , Mollard, V. , … Siden‐Kiamos, I. (2018). Sequential membrane rupture and vesiculation during Plasmodium berghei gametocyte egress from the red blood cell. Scientific Reports, 8, 3543 10.1038/s41598-018-21801-3 - DOI - PMC - PubMed
-
- Billker, O. , Dechamps, S. , Tewari, R. , Wenig, G. , Franke‐Fayard, B. , & Brinkmann, V. (2004). Calcium and a calcium‐dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell, 117(4), 503–514. - PubMed
-
- Billker, O. , Lindo, V. , Panico, M. , Etienne, A. E. , Paxton, T. , Dell, A. , … Morris, H. R. (1998). Identification of xanthurenic acid as the putative inducer of malaria development in the mosquito. Nature, 392, 289–292. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

