Safety and Vision Outcomes of Subretinal Gene Therapy Targeting Cone Photoreceptors in Achromatopsia: A Nonrandomized Controlled Trial
- PMID: 32352493
- PMCID: PMC7193523
- DOI: 10.1001/jamaophthalmol.2020.1032
Safety and Vision Outcomes of Subretinal Gene Therapy Targeting Cone Photoreceptors in Achromatopsia: A Nonrandomized Controlled Trial
Abstract
Importance: Achromatopsia linked to variations in the CNGA3 gene is associated with day blindness, poor visual acuity, photophobia, and involuntary eye movements owing to lack of cone photoreceptor function. No treatment is currently available.
Objective: To assess safety and vision outcomes of supplemental gene therapy with adeno-associated virus (AAV) encoding CNGA3 (AAV8.CNGA3) in patients with CNGA3-linked achromatopsia.
Design, setting, and participants: This open-label, exploratory nonrandomized controlled trial tested safety and vision outcomes of gene therapy vector AAV8.CNGA3 administered by subretinal injection at a single center. Nine patients (3 per dose group) with a clinical diagnosis of achromatopsia and confirmed biallelic disease-linked variants in CNGA3 were enrolled between November 5, 2015, and September 22, 2016. Data analysis was performed from June 6, 2017, to March 12, 2018.
Intervention: Patients received a single unilateral injection of 1.0 × 1010, 5.0 × 1010, or 1.0 × 1011 total vector genomes of AAV8.CNGA3 and were followed up for a period of 12 months (November 11, 2015, to October 10, 2017).
Main outcomes and measures: Safety as the primary end point was assessed by clinical examination of ocular inflammation. Systemic safety was assessed by vital signs, routine clinical chemistry testing, and full and differential blood cell counts. Secondary outcomes were change in visual function from baseline in terms of spatial and temporal resolution and chromatic, luminance, and contrast sensitivity throughout a period of 12 months after treatment.
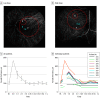
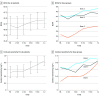
Results: Nine patients (mean [SD] age, 39.6 [11.9] years; age range, 24-59 years; 8 [89%] male) were included in the study. Baseline visual acuity letter score (approximate Snellen equivalent) ranged from 34 (20/200) to 49 (20/100), whereas baseline contrast sensitivity log scores ranged from 0.1 to 0.9. All 9 patients underwent surgery and subretinal injection of AAV8.CNGA3 without complications. No substantial safety problems were observed during the 12-month follow-up period. Despite the congenital deprivation of cone photoreceptor-mediated vision in achromatopsia, all 9 treated eyes demonstrated some level of improvement in secondary end points regarding cone function, including mean change in visual acuity of 2.9 letters (95% CI, 1.65-4.13; P = .006, 2-sided t test paired samples). Contrast sensitivity improved by a mean of 0.33 log (95% CI, 0.14-0.51 log; P = .003, 2-sided t test paired samples).
Conclusions and relevance: Subretinal gene therapy with AAV8.CNGA3 was not associated with substantial safety problems and was associated with cone photoreceptor activation in adult patients, as reflected by visual acuity and contrast sensitivity gains.
Trial registration: ClinicalTrials.gov Identifier: NCT02610582.
Conflict of interest statement
Figures

References
-
- Blackwell HR, Blackwell OM. Rod and cone receptor mechanisms in typical and atypical congenital achromatopsia. Vision Res. 1961;1:62-107. doi:10.1016/0042-6989(61)90022-0 - DOI

